Page 97 - Pipeline Risk Management Manual Ideas, Techniques, and Resources
P. 97
Scoring the corrosion potential 4/75
This part of the risk assessment will apply to metallic pipe cell can be established between the two metals. Dissimilar soils
material that is buried or submerged. If the pipeline being eval- with differences in concentrations of ions, oxygen, or moisture
uated is not vulnerable to subsurface corrosion, as would be the can also set up anodic and cathodic regions on the pipe surface.
case for a plastic pipeline or a totally aboveground pipeline, the Corrosion cells ofthis type are called concentration cells. When
evaluator should use the previous two sections and any other these cells are established, the anodic region will experience
pertinent factors to assess the corrosion risk. active corrosion. The severity of this corrosion is dictated by
Of the three categories of corrosion, this is usually consid- variables such as the conductivity of the soil (electrolyte) and the
ered to be the most complex. Several corrosion mechanisms relative electronegativities ofthe anode and cathode.
can be at work in the case ofburiedmetals. This situation is fur- Common industry practice is to employ a two-part defense
ther complicated by the fact that corrosion activity is normally against galvanic corrosion of a pipeline. The first line of
deduced only from indirect evidence4irect observation is a defense is a coating over the pipeline. This is designed to isolate
rather limited option. the metal from the electrolyte. If this coating is perfect, the gal-
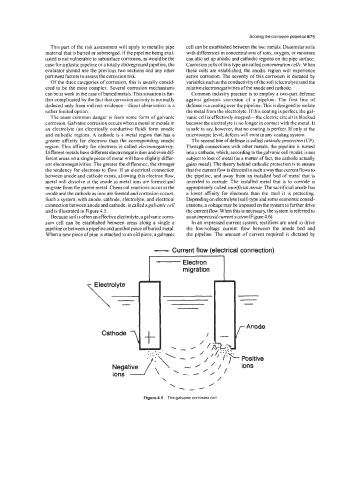
The most common danger is from some form of galvanic vanic cell is effectively stopped-the electric circuit is blocked
corrosion. Galvanic corrosion occurs when a metal or metals in because the electrolyte is no longer in contact with the metal. It
an electrolyte (an electrically conductive fluid) form anodic is safe to say, however, that no coating is perfect. If only at the
and cathodic regions. A cathode is a metal region that has a microscopic level, defects will exist in any coating system.
greater affinity for electrons than the corresponding anodic The second line of defense is called cathodicprofecfion (CP).
region. This affinity for electrons is called electronegativity. Through connections with other metals, the pipeline is turned
Different metals have different electronegativities and even dif- into a cathode, which, according to the galvanic cell model, is not
ferent areas on a single piece of metal will have slightly differ- subject to loss of metal (as a matter of fact, the cathode actually
ent electronegativities. The greater the difference, the stronger gains metal). The theory behind cathodic protection is to ensure
the tendency for electrons to flow. If an electrical connection that the current flow is directed in such a way that current flows to
between anode and cathode exists. allowing this electron flow, the pipeline, and away from an installed bed of metal that is
metal will dissolve at the anode as metal ions are formed and intended to corrode. The installed metal that is to corrode is
migrate from the parent metal. Chemical reactions occur at the appropriately called sacrificial anode. The sacrificial anode has
anode and the cathode as ions are formed and corrosion occurs. a lower affinity for electrons than the steel it is protecting.
Such a system, with anode, cathode, electrolyte, and electrical Depending on electrolyte (soil) type and some economic consid-
connection between anode and cathode, is called agalvanic cell erations, a voltage may be imposed on the system to further drive
and is illustrated in Figure 4.5. the current flow. When this is necessary, the system is referred to
Because soil is often an effective electrolyte, agalvanic corro- as an impressed current system (Figure 4.6).
sion cell can be established between areas along a single a In an impressed current system, rectifiers are used to drive
pipeline or between a pipeline and another piece ofburiedmetal. the low-voltage current flow between the anode bed and
When a new piece of pipe is attached to an old piece, a galvanic the pipeline. The amount of current required is dictated by
- Current flow (electrical connection)
migration
Electrolyte
Negati \ \ - 1, ’ /,‘ ions
ions ‘-/
/
\ -- /
/
\ I
Figure 4.5 The galvanic corrosion cell