Page 251 - Pressure Swing Adsorption
P. 251
' "
228
PRESSURE SWING ADSORPTION PSA PROCESSES 229
100
{o) Oz Concentrot1on
;; 95~ 0 (I [ rnnax PROCfSS /J970)
"
u " " - /<d$orbenl ,., '"
~•I'· uctor
e ,,_ -- Bed~ • '"
a_ - Valve~ '
"
" ..
Prenur" Ranqe (at.o}
a_ 90>- 0, Recoe~r:r {1') ].3/l.0 !. J/0.2
"
rOw~r (kJ/o,o\e product)
I - lycle Ti.,e (llllnsl "
~ork,n9 Lapacity ot ad~orbeet '
.S ( .. olu product/~g: ocle) 0.0lll 0.06
85 Msorbenl Inventory (tons)
1
0 for 200 Ii o, /hr production '"
;;,
80>- Product (l at,.) Air (I.I atmJo---P,oduct (l .tmj
0.09 11ahs I IIIOle 0.16 IIIOleS
O,: 90l: o.: 21'.I: 0,: 9!t;
' ' ' ' . • 0 11;: IOl. N,: 791: I N;: !l":.
( b) 0 2 Recovery and Power
20 o'= 00 llnle 1/aHe I
6
0 0 0.91 •mies 0.64 moles
02: 10;
0,: 8:t
15 r ? II,: 86'4 II,: 92'l
0 ~ 0 ~:-125~
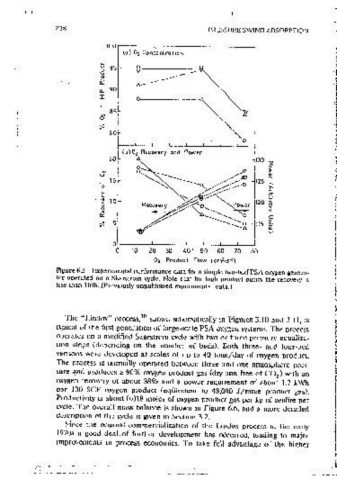
Figure 6.6 Compnnson of LINDOX PSA oxygen process with a modern two-bed
~,f{i~ ?: VSA process. Figures arc approximate est1m<1tcs based on mformatmn from a vanccv
of sources.
~~-120!
5 ~□ -115;::;.·
~
® 6 "'
a,'---'----'-. _ __,_ __ _,_·---'-·--''-----'-'---' select1v1ty and capacity of the "second generation" adsorbents requJres
0 10 20 30 ,o' 50 60 70 80 desomt1on at subatmosphenc pressure, necess1tatmg :the use of a vacuum
02 Product Flow (crn3,s·l) swmg or pressure/vacuum swmg cycle. This leads to a substantial reduct10n
in the adsorbent inventory relative to the traditiOnai p·rcssure swing process
Figure 6.5 Experimental performance data for a simple two-bed PSA oxygen genera-
tor operated on a Skarstrom cycle. Note that for high product punty the recovery 1s but at the cost of a somewhat more comolex cycle with both a feed
less than 10%. (Prevmusly unpublished expenmental ctata.) compressor and a vacuum pump. Since the valves and ·pipmg must be larger
for vacuum ooerat10n, there ts some penalty m capital cost, but this 1s more
than offset by the reduction m actsorbenl mventory and power cost.
Modern VSA oxygen systems generally operate belween about J.5-2.5
10
The "Undox" process, shown schematically m Figures 3.10 and 3.11, 1s atmosoheres on the high-oressure side with ctesorpuon at 0.25-0.35 atmo-
typical of the first generation of large-scale PSA oxygen systems. The process spheres so the pressure ratio ts substantially greater than for the earlier
operates on a modified Skarstrom cycle with two or three pressure eaualiza• supra-atmospheric pressure processes. The cycle 1s b(!lsical!v similar to the
11011 slcps (depending on the number of beds), Both three- and four-bed ongmai Air Liouide cycle (Figure 3.12) but with the addiu,m of a pressure
versions were deveioped at scales of up to 40 tons/day of oxygen product. equalization step. The adsorbent (Ca.X") is OOJsoned by traces of water or
The orocess 1s normally operated between three and one atmosphere ores- carbon dioxide so ore-beds are often mcluded to remove these comPonents.
sure and produces a 90% oxygen product gas (dry and free of CO ) with an
2 The use of vacuum desorot1on elimmates the requirement for mul liole-bed
oxygen recovery of about 38% and a power requirement of about 1.7 kWh systems to recover the energy of compression. As a result the more modern
per I 00 SCF oxygen product (eouivaient to 48,000 J /mole product gas). VPSA oxygen processes generally use only two beds rather than the three or
Productivity 1s about 0.018 moles of oxygen product gas per kg of zeolite per four beds of the earlier large.;.scale pressure swmg processes, with conseauent
cvcie. The overall mass balance 1s shown in Figure 6.6, and a more detailed reduction of caDJtal costs. An approximate· performance cornpanson between
Uescnption of the cycie is given rn Sectmn 3.2.
a modern VSA oxygen process and the onginal LJNDOX process 1s given m
Sinc·c the origmal commercialization of the Lindox process In the eariy Figure 6.6. Power reauirements are similar, but the adlmrbent inventory has
1970s n -good deat. of further development has occurred, leading to major been reduced to about 20% of that required in the first generation processes
improvements m process economics. To take full advantage of the higher as a result of improved adsorbent capacity, reduced- cvcle time, and the