Page 253 - Pressure Swing Adsorption
P. 253
-,
i
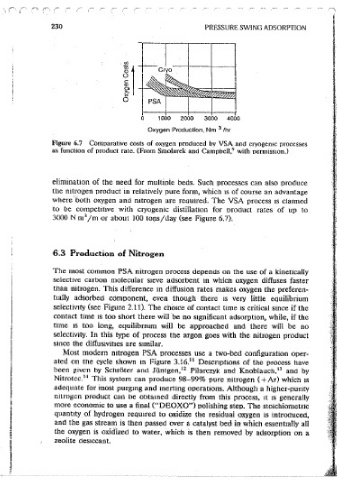
230 PRESSURE SWING ADSORPTION PSA PROCESSES 231
I
Crvo
11
0
0 1000 2000 3000 4000
3
Oxygen Production, Nm /hr
Figure 6.7 Comparative costs of oxygen produced bv VSA and cryogenic processes
9
as function of product rate. (From Smolarek and Campbell, with oermiss1on.)
elimination of the need for multioie beds. Such processes can also produce
the mtrogen product in relatively pure form, which 1s of course an advantage
where· both oxygen and mtrogen are required. The VSA process 1s clatmed
to be competitive with cryogenic distillation for oroduct rates of up to
3
3000 N m /m or about JOO to~s/day (see Figure 6.7).
6.3 Production of Nitrogen Figure 6.8 Small-scale skid-mounted PSA nitrogen generator. (Courtesy of Nltrotcc,
Inc,1 4 )
The most common PSA nitrogen orocess depends on the use of a kinetically
selective carbon molecular sieve adsorbent in which oxygen diffuses faster
tMm nitrogen. This difference in diffusion rates makes oxygen the preferen~ A schematic diagram of the Bergbau Forschung process 1s shown in Figure
t1ally adsorbed component, even though there 1s very little equilibrium 3.15, and a typical small-scale umt, produced and marketed by Nitrotec, 1s
selectivity (see Figure 2.11). The choice of contact time 1s critical smce if the shown m Figure 6.8. The Process operates between 6-8 and 1 atmospheres
contact time 1s too short there will be no significant adsorption, while, if the oressure. Standard umts are produced in varying sizes with mtrogen product
time 1s too long, equilibnum will be approached and there will be no rates from 60 to 60,000 SCFH. A typical overall mass balance 1s shown m
selectivity. In this type of process the argon goes with the nitrogen product Figure 6.9, and representative performance data are summanzed m Table
since the diffus1vit1es are similar. 6.1. The reduction in throughout and the corresoonding reduction m recov-
Most modern mtrogen PSA processes use a two-bed configuration oper- ery and the increase in the specific energy requirement with mcreasmg punty
ated on the cycle shown m Figure 3.16. 11 Descriptions of the process have of the nitrogen product are cleariy apparent. The economics of this type of
been given by Schr0ter and Jiintgen, 12 Pilarczyk and Knoblauch, and by process are at thelf best m the 98-99% purity range at product rates of
13
14
Nitrotcc. This system can produce 98-99% pure nitrogen (+Ar) which 1s 200-800 Nm'/h, although much larger urn ls (up to 4800 Nm'/hr or ahout
15
adequate for lllost purgmg and merting operations. Although a higher~ountv 150 tons/day) nave been built. Typical operatmg costs (assummg electnc
nitrogen product can be obtained directly from this process, 1t 1s generally vower at $0.05 per kwh) are about $0.30-0.40 per 1000 SCF (28 Nm·') for
more economic to use a final ("DEOXO") polishing step. The stoichiometric 98% and 99.5% ounty resoect1vely (1992· figures)*. At this levei costs are
quantity. of hydrogen reomred to oxidize the residual oxygen is introduced, comparable with a cryogenic umt over a faJTiy wide :range and only at the
and the gas stream Is then passed over a catalyst bed in which essentially all
the oxygen 1s oxidized to water, which is then removed by adsorption on a
zeolite desiccant.
*These cost estimates were kindly provided by Mr. Herbert Retnhold of Nitrotec.