Page 81 - Principles of Catalyst Development
P. 81
68 CHAPTER 4
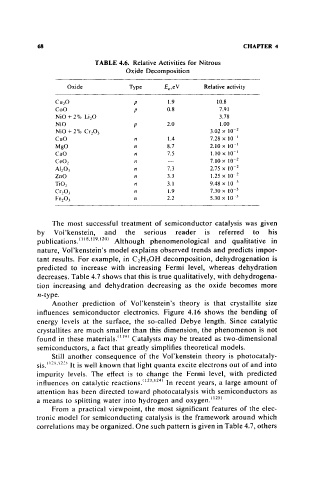
TABLE 4.6. Relative Activities for Nitrous
Oxide Decomposition
Oxide Type E,.eV Relative activity
Cu 20 p 1.9 10.8
CoO P 0.8 7.91
NiO + 2% Li,O 3.78
NiO P 2.0 1.00
NiO + 2% Cr20) 3.02 x 10- 2
CuO n 1.4 7.28 x 10'
MgO n 8.7 2.10 x 10- 1
CaO n 7.5 1.10x 10- 1
CeO, n 7.10 X 10- 2
Al,O, n 7.3 2.75 x 10- 2
ZoO n 3.3 1.25 x 10. 2
TiO, n 3.1 9.48 x 10 3
Cr,O, n 1.9 7.30 x 10- 3
Fe203 n 2.2 5.30x 10- 3
The most successful treatment of semiconductor catalysis was given
by Vol'kenstein, and the serious reader is referred to his
publications. (118.119.120) Although phenomenological and qualitative in
nature, Vol'kenstein's model explains observed trends and predicts impor-
tant results. For example, in C 2HsOH decomposition, dehydrogenation is
predicted to increase with increasing Fermi level, whereas dehydration
decreases. Table 4.7 shows that this is true qualitatively, with dehydrogena-
tion increasing and dehydration decreasing as the oxide becomes more
n-type.
Another prediction of Vol'kenstein's theory is that crystallite size
influences semiconductor electronics. Figure 4.16 shows the bending of
energy levels at the surface, the so-called Oebye length. Since catalytic
crystallites are much smaller than this dimension, the phenomenon is not
found in these materials.(119) Catalysts may be treated as two-dimensional
semiconductors, a fact that greatly simplifies theoretical models.
Still another consequence of the Vol'kenstein theory is photocataly-
sis.ml.l m It is well known that light quanta excite electrons out of and into
impurity levels. The effect is to change the Fermi level, with predicted
influences on catalytic reactions.( 123.124) In recent years, a large amount of
attention has been directed toward photocatalysis with semiconductors as
a means to splitting water into hydrogen and oxygenY2S)
From a practical viewpoint, the most significant features of the elec-
tronic model for semiconducting catalysis is the framework around which
correlations may be organized. One such pattern is given in Table 4.7, others