Page 82 - Principles of Catalyst Development
P. 82
CATALYTIC MATERIALS 69
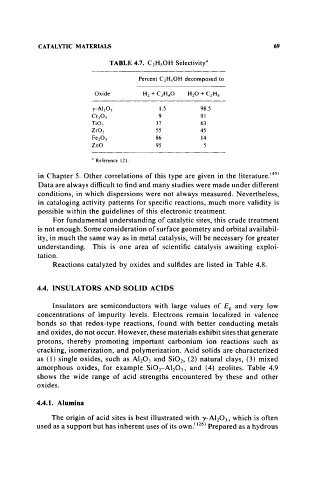
TABLE 4.7. C 2 HsOH SelectivityU
Percent C 2 HsOH decomposed to
Oxide H2 + C2H 4O H2 0 + C2H 4
'Y- AI 2 0 3 1.5 98.5
Cr2 0 3 9 91
Ti02 37 63
Zr02 55 45
Fe2 0 3 86 14
ZnO 95 5
u Reference 121.
in Chapter 5. Other correlations of this type are given in the literature.(45)
Data are always difficult to find and many studies were made under different
conditions, in which dispersions were not always measured. Nevertheless,
in cataloging activity patterns for specific reactions, much more validity is
possible within the guidelines of this electronic treatment.
For fundamental understanding of catalytic sites, this crude treatment
is not enough. Some consideration of surface geometry and orbital availabil-
ity, in much the same way as in metal catalysis, will be necessary for greater
understanding. This is one area of scientific catalysis awaiting exploi-
tation.
Reactions catalyzed by oxides and sulfides are listed in Table 4.8.
4.4. INSULATORS AND SOLID ACIDS
Insulators are semiconductors with large values of Eg and very low
concentrations of impurity levels. Electrons remain localized in valence
bonds so that redox-type reactions, found with better conducting metals
and oxides, do not occur. However, these materials exhibit sites that generate
protons, thereby promoting important carbonium ion reactions such as
cracking, isomerization, and polymerization. Acid solids are characterized
as (1) single oxides, such as Al 20 3 and Si0 2 , (2) natural clays, (3) mixed
amorphous oxides, for example Si0 2-AI 20 3 , and (4) zeolites. Table 4.9
shows the wide range of acid strengths encountered by these and other
oxides.
4.4.1. Alumina
The origin of acid sites is best illustrated with ')'-AI 20), which is often
used as a support but has inherent uses of its own.(l26) Prepared as a hydrous