Page 87 - Principles of Catalyst Development
P. 87
74 CHAPTER 4
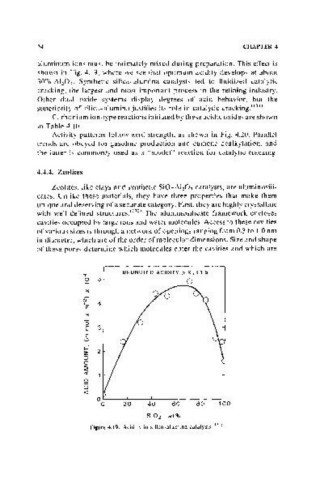
aluminum ions must be intimately mixed during preparation. This effect is
shown in Fig. 4.19, where we see that optimum acidity develops at about
30% AI~Ol' Synthetic silica-alumina catalysts led to fluidized catalytic
cracking, the largest and most important process in the refining industry.
Other dual oxide systems display degrees of acid behavior, but the
superiority of silica-alumina justifies its role in catalytic cracking. mll
Carbonium ion-type reactions initiated by these acidic oxides are shown
in Table 4.10.
Activity patterns follow acid strength, as shown in Fig. 4.20. Parallel
trends are obeyed for gasoline production and cumene dealkylation, and
the latter is commonly used as a "model" reaction for catalytic cracking.
4.4.4. Zeolites
Zeolites, like clays and synthetic SiO-AI 2 0 3 catalysts, are aluminosili-
cates. Unlike these materials, they have three properties that make them
unique and deserving of a separate category. First, they are highly crystalline
with well-defined structures.(1321 The aluminosilicate framework encloses
cavities occupied by large ions and water molecules. Access to these cavities
of various sizes is through a network of openings ranging from 0.3 to 1.0 nm
in diameter, which are of the order of molecular dimensions. Size and shape
of these pores determine which molecules enter the cavities and which are
BRONS TED ACIDITY p Ka ~ 1.5
'-t
o 5 o
><
o
E 3
E
~- 2
;:)
°
~
«
a
o
«
20 40 60 80 100
Si0 2 , wt%
Figure 4.19. Acidity in silica-alumina catalysts.'!3I)