Page 84 - Principles of Catalyst Development
P. 84
CATALYTIC MATERIALS 71
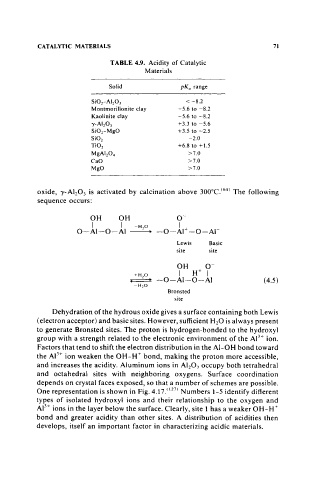
TABLE 4.9. Acidity of Catalytic
Materials
Solid pKa range
SiOr AI 20 3 < -8.2
Montmorillonite clay -5.6 to -8.2
Kaolinite clay -5.6 to -8.2
r- A1 203 + 3.3 to -5.6
SiOrMgO +3.5 to -2.5
Si0 2 -2.0
Ti0 2 +6.8 to +1.5
MgAI 20 4 >7.0
CaO >7.0
MgO >7.0
oxide, "y-AI203 is activated by calcination above 300°c. (64 ) The following
sequence occurs:
OH OH 0-
I I -H,O I
O-Al-O-Al -- -O-Al+ -O-Al-
Lewis Basic
site site
OH 0-
I H+ I
-O-Al-O-Al (4.5)
Bronsted
site
Dehydration of the hydrous oxide gives a surface containing both Lewis
(electron acceptor) and basic sites. However, sufficient H 20 is always present
to generate Bronsted sites. The proton is hydrogen-bonded to the hydroxyl
group with a strength related to the electronic environment of the AIH ion.
Factors that tend to shift the electron distribution in the Al-OH bond toward
the AI3+ ion weaken the OH-H+ bond, making the proton more accessible,
and increases the acidity. Aluminum ions in Al 2 0, occupy both tetrahedral
and octahedral sites with neighboring oxygens. Surface coordination
depends on crystal faces exposed, so that a number of schemes are possible.
One representation is shown in Fig. 4.17.(127) Numbers 1-5 identify different
types of isolated hydroxyl ions and their relationship to the oxygen and
AlH ions in the layer below the surface. Clearly, site 1 has a weaker OH-H+
bond and greater acidity than other sites. A distribution of acidities then
develops, itself an important factor in characterizing acidic materials.