Page 241 - Process Equipment and Plant Design Principles and Practices by Subhabrata Ray Gargi Das
P. 241
9.3 Representation of equilibrium 241
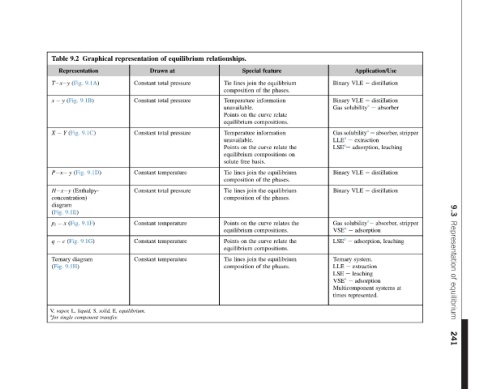
Application/Use distillation e distillation e absorber e Gas solubility a e absorber, stripper extraction leaching adsorption, distillation e distillation e stripper absorber, solubility a e adsorption leaching adsorption, system. extraction leaching adsorption at systems represented.
Binary VLE VLE Binary solubility a Gas e LLE a LSE a e VLE Binary VLE Binary Gas e VSE a e LSE a Ternary e LLE e LSE e VSE a Multicomponent times
equilibrium phases. relate the relate on equilibrium phases. equilibrium phases. the relates the relate equilibrium phases.
feature the the information curve compositions. information curve compositions the the the the curve compositions. curve compositions. the the
Special join lines of the on the on basis. free join lines of join lines of the on the on join lines of
relationships. Tie composition Temperature unavailable. Points equilibrium Temperature unavailable. Points equilibrium solute Tie composition Tie composition Points equilibrium Points equilibrium Tie composition
equilibrium pressure pressure pressure pressure
of at Drawn total total total temperature total temperature temperature temperature
representation Constant Constant Constant Constant Constant Constant Constant Constant equilibrium.
Graphical 9.1A) 9.1B) 9.1C) 9.1D) 9.1F) 9.1G) E, solid, S, transfer. component
9.2 Representation (Fig. (Fig. (Fig. (Fig. y (Enthalpy- concentration) 9.1E) (Fig. (Fig. diagram 9.1H) liquid, L,
Table T x y y x Y X P x H x y diagram (Fig. x p i c q Ternary (Fig. vapor, V, a for single