Page 39 - Reservoir Formation Damage
P. 39
Mineralogy and Mineral Sensitivity of Petroleum-Bearing Formations 23
d(001)-spadng
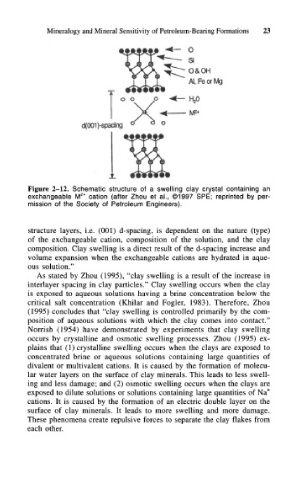
Figure 2-12. Schematic structure of a swelling clay crystal containing an
exchangeable M z+ cation (after Zhou et al., ©1997 SPE; reprinted by per-
mission of the Society of Petroleum Engineers).
structure layers, i.e. (001) d-spacing, is dependent on the nature (type)
of the exchangeable cation, composition of the solution, and the clay
composition. Clay swelling is a direct result of the d-spacing increase and
volume expansion when the exchangeable cations are hydrated in aque-
ous solution."
As stated by Zhou (1995), "clay swelling is a result of the increase in
interlayer spacing in clay particles." Clay swelling occurs when the clay
is exposed to aqueous solutions having a brine concentration below the
critical salt concentration (Khilar and Fogler, 1983). Therefore, Zhou
(1995) concludes that "clay swelling is controlled primarily by the com-
position of aqueous solutions with which the clay comes into contact."
Norrish (1954) have demonstrated by experiments that clay swelling
occurs by crystalline and osmotic swelling processes. Zhou (1995) ex-
plains that (1) crystalline swelling occurs when the clays are exposed to
concentrated brine or aqueous solutions containing large quantities of
divalent or multivalent cations. It is caused by the formation of molecu-
lar water layers on the surface of clay minerals. This leads to less swell-
ing and less damage; and (2) osmotic swelling occurs when the clays are
+
exposed to dilute solutions or solutions containing large quantities of Na
cations. It is caused by the formation of an electric double layer on the
surface of clay minerals. It leads to more swelling and more damage.
These phenomena create repulsive forces to separate the clay flakes from
each other.