Page 37 - Reservoir Formation Damage
P. 37
Mineralogy and Mineral Sensitivity of Petroleum-Bearing Formations 21
contrast, the larger cations present in the aqueous solution tend to dif-
fuse into clays because there are more of the larger cations in the solu-
tion compared to the clays. Because larger cations cannot fit into the
interplanar gap depleted by K + cations, the edges of the friable mica
flakes break off in small pieces as depicted in Figure 2-10. By a differ-
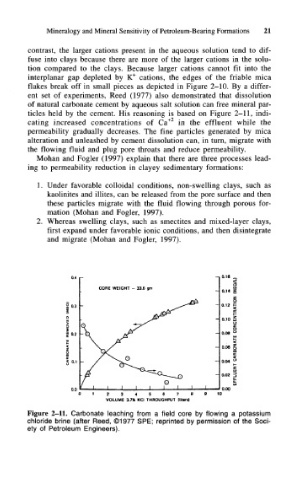
ent set of experiments, Reed (1977) also demonstrated that dissolution
of natural carbonate cement by aqueous salt solution can free mineral par-
ticles held by the cement. His reasoning is based on Figure 2-11, indi-
cating increased concentrations of Ca +2 in the effluent while the
permeability gradually decreases. The fine particles generated by mica
alteration and unleashed by cement dissolution can, in turn, migrate with
the flowing fluid and plug pore throats and reduce permeability.
Mohan and Fogler (1997) explain that there are three processes lead-
ing to permeability reduction in clayey sedimentary formations:
1. Under favorable colloidal conditions, non-swelling clays, such as
kaolinites and illites, can be released from the pore surface and then
these particles migrate with the fluid flowing through porous for-
mation (Mohan and Fogler, 1997).
2. Whereas swelling clays, such as smectites and mixed-layer clays,
first expand under favorable ionic conditions, and then disintegrate
and migrate (Mohan and Fogler, 1997).
0.16 _
3
CORE WEIGHT - 33.6 yn 1U
0.14 5
O
20.3 0.12 P
s tc
t-
0.10 £
0.2
< 0.1 0.04
0.02 3
0.00
2 3 4 5 6 7 8 10
VOLUME 3.7* KCI THROUGHPUT (literj)
Figure 2-11. Carbonate leaching from a field core by flowing a potassium
chloride brine (after Reed, ©1977 SPE; reprinted by permission of the Soci-
ety of Petroleum Engineers).