Page 104 - Science at the nanoscale
P. 104
9:10
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Surfaces at the Nanoscale
94
1 nm 3 ch05
1 cm
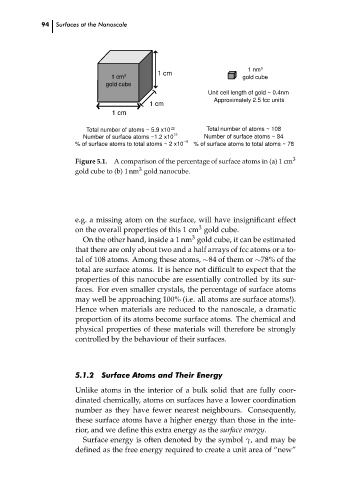
1 cm 3 gold cube
gold cube
Unit cell length of gold ~ 0.4nm
Approximately 2.5 fcc units
1 cm
1 cm
Total number of atoms ~ 5.9 x10 22 Total number of atoms ~ 108
Number of surface atoms ~1.2 x10 15 Number of surface atoms ~ 84
_
% of surface atoms to total atoms ~ 2 x10 6 % of surface atoms to total atoms ~ 78
Figure 5.1. A comparison of the percentage of surface atoms in (a) 1 cm 3
3
gold cube to (b) 1 nm gold nanocube.
e.g. a missing atom on the surface, will have insignificant effect
3
on the overall properties of this 1 cm gold cube.
3
On the other hand, inside a 1 nm gold cube, it can be estimated
that there are only about two and a half arrays of fcc atoms or a to-
tal of 108 atoms. Among these atoms, ∼84 of them or ∼78% of the
total are surface atoms. It is hence not difficult to expect that the
properties of this nanocube are essentially controlled by its sur-
faces. For even smaller crystals, the percentage of surface atoms
may well be approaching 100% (i.e. all atoms are surface atoms!).
Hence when materials are reduced to the nanoscale, a dramatic
proportion of its atoms become surface atoms. The chemical and
physical properties of these materials will therefore be strongly
controlled by the behaviour of their surfaces.
5.1.2 Surface Atoms and Their Energy
Unlike atoms in the interior of a bulk solid that are fully coor-
dinated chemically, atoms on surfaces have a lower coordination
number as they have fewer nearest neighbours. Consequently,
these surface atoms have a higher energy than those in the inte-
rior, and we define this extra energy as the surface energy.
Surface energy is often denoted by the symbol γ, and may be
defined as the free energy required to create a unit area of “new”