Page 107 - Science at the nanoscale
P. 107
9:10
June 9, 2009
the lowest Gibbs free energy. In order to lower the free energy,
some surface processes such as surface relaxation or reconstruc-
tion will occur naturally. RPS: PSP0007 - Science-at-Nanoscale 5.1. Surface Energy 97 ch05
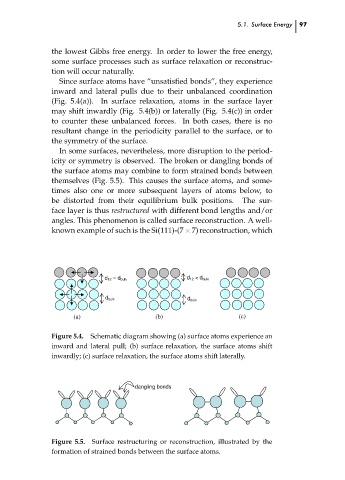
Since surface atoms have “unsatisfied bonds”, they experience
inward and lateral pulls due to their unbalanced coordination
(Fig. 5.4(a)). In surface relaxation, atoms in the surface layer
may shift inwardly (Fig. 5.4(b)) or laterally (Fig. 5.4(c)) in order
to counter these unbalanced forces. In both cases, there is no
resultant change in the periodicity parallel to the surface, or to
the symmetry of the surface.
In some surfaces, nevertheless, more disruption to the period-
icity or symmetry is observed. The broken or dangling bonds of
the surface atoms may combine to form strained bonds between
themselves (Fig. 5.5). This causes the surface atoms, and some-
times also one or more subsequent layers of atoms below, to
be distorted from their equilibrium bulk positions. The sur-
face layer is thus restructured with different bond lengths and/or
angles. This phenomenon is called surface reconstruction. A well-
known example of such is the Si(111)-(7 × 7) reconstruction, which
d 12 = d bulk d 12 < d bulk
d bulk d bulk
(a) (b) (c)
Figure 5.4. Schematic diagram showing (a) surface atoms experience an
inward and lateral pull; (b) surface relaxation, the surface atoms shift
inwardly; (c) surface relaxation, the surface atoms shift laterally.
dangling bonds
Figure 5.5. Surface restructuring or reconstruction, illustrated by the
formation of strained bonds between the surface atoms.