Page 111 - Science at the nanoscale
P. 111
RPS: PSP0007 - Science-at-Nanoscale
9:10
June 9, 2009
5.2. Surface Reactivity and Catalysis
In general, we may divide the catalytic surface reaction into sev-
eral consecutive steps: (1) diffusion of reactants to the surface;
(2) adsorption of reactants at the surface; (3) chemical transforma- 101 ch05
tion of the adsorbed molecule, (4) reaction on the surface; (4) des-
orption of products from the surface; (5) diffusion of products
away from the surface. Suppose any of these steps has a much
lower rate constant than all the others, it will become the rate
determining step and control the overall rate of reaction. Thus,
while higher surface area causes smaller particles to react faster,
the situation is less straight-forward for heterogeneous solid cata-
lytic reactions.
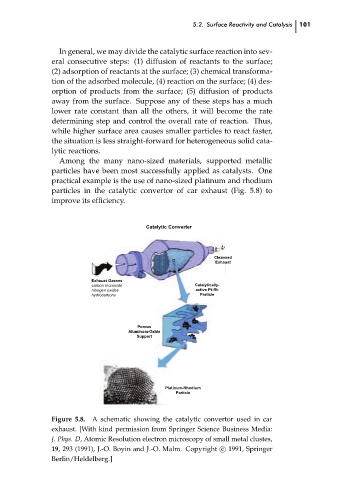
Among the many nano-sized materials, supported metallic
particles have been most successfully applied as catalysts. One
practical example is the use of nano-sized platinum and rhodium
particles in the catalytic convertor of car exhaust (Fig. 5.8) to
improve its efficiency.
Catalytic Converter
Cleansed
Exhaust
Exhaust Gasses
carbon monoxide Catalytically-
nitrogen oxides active Pt-Rh
hydrocarbons Particle
Porous
Aluminum-Oxide
Support
Platinum-Rhodium
Particle
Figure 5.8. A schematic showing the catalytic convertor used in car
exhaust. [With kind permission from Springer Science Business Media:
J. Phys. D, Atomic Resolution electron microscopy of small metal clustes,
19, 293 (1991), J.-O. Boyin and J.-O. Malm. Copyright c
1991, Springer
Berlin/Heldelberg.]