Page 110 - Science at the nanoscale
P. 110
9:10
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Surfaces at the Nanoscale
100
5.2 SURFACE REACTIVITY AND CATALYSIS
In chemical reactions involving a solid material, the surface area ch05
to volume ratio plays an important role in the reactivity. This is
analogous to the situation where finely crushed ice melts faster
than ice cubes. Materials with higher surface area are expected to
react more readily because more surface sites are available to react.
A famous historical example is the destructive explosion caused
by a spark and flour dust in the “Great Mill Disaster” accident in
5
1878; while grain is not typically flammable, grain dust becomes
explosive due to its extremely high surface energy. Thermody-
namically, a high surface area provides a strong “driving force”
that speeds up processes in the quest to minimise free energy.
High surface area can be achieved either by using materials of
very small sizes or materials that possess highly porous struc-
6
tures. In the latter, microporous materials such as zeolites have
played an important role in heterogeneous catalysis. A catalyst
speeds up reactions by providing an alternative reaction pathway
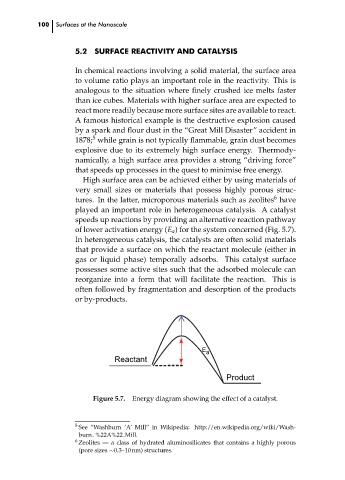
of lower activation energy (E a ) for the system concerned (Fig. 5.7).
In heterogeneous catalysis, the catalysts are often solid materials
that provide a surface on which the reactant molecule (either in
gas or liquid phase) temporally adsorbs. This catalyst surface
possesses some active sites such that the adsorbed molecule can
reorganize into a form that will facilitate the reaction. This is
often followed by fragmentation and desorption of the products
or by-products.
E a
Reactant
Product
Figure 5.7. Energy diagram showing the effect of a catalyst.
5 See “Washburn ‘A’ Mill” in Wikipedia: http://en.wikipedia.org/wiki/Wash-
burn %22A%22 Mill.
6 Zeolites — a class of hydrated aluminosilicates that contains a highly porous
(pore sizes ∼0.3–10 nm) structures.