Page 113 - Science at the nanoscale
P. 113
RPS: PSP0007 - Science-at-Nanoscale
9:10
June 9, 2009
5.3. Surface stabilisation
Michael Faraday called this system “sols”, and demonstrated in
10
his lecture the vivid ruby red colour of the gold sols he prepared.
Indeed Faraday stated in this historical paper that “known phenom- 103 ch05
ena seemed to indicate that a mere variation in the size of particles gave
rise to a variety of resultant colours”. This is probably the earliest
scientific expression of the “size effect” referred to in Chapter 3.
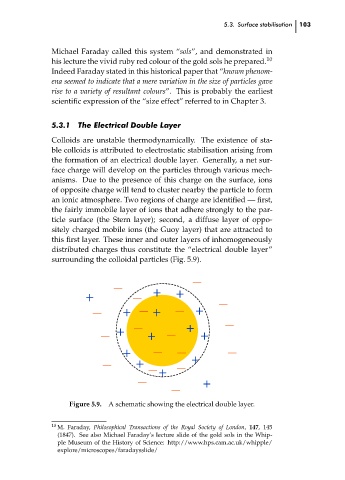
5.3.1 The Electrical Double Layer
Colloids are unstable thermodynamically. The existence of sta-
ble colloids is attributed to electrostatic stabilisation arising from
the formation of an electrical double layer. Generally, a net sur-
face charge will develop on the particles through various mech-
anisms. Due to the presence of this charge on the surface, ions
of opposite charge will tend to cluster nearby the particle to form
an ionic atmosphere. Two regions of charge are identified — first,
the fairly immobile layer of ions that adhere strongly to the par-
ticle surface (the Stern layer); second, a diffuse layer of oppo-
sitely charged mobile ions (the Guoy layer) that are attracted to
this first layer. These inner and outer layers of inhomogeneously
distributed charges thus constitute the “electrical double layer”
surrounding the colloidal particles (Fig. 5.9).
_
_
+ _ + + _
_ + _ + _ +
_ + _
_ + + _ +
+ _ _ _
_ + _ _ +
_ +
_ +
Figure 5.9. A schematic showing the electrical double layer.
10 M. Faraday, Philosophical Transactions of the Royal Society of London, 147, 145
(1847). See also Michael Faraday’s lecture slide of the gold sols in the Whip-
ple Museum of the History of Science: http://www.hps.cam.ac.uk/whipple/
explore/microscopes/faradaysslide/