Page 114 - Science at the nanoscale
P. 114
9:10
June 9, 2009
Surfaces at the Nanoscale
104
V RPS: PSP0007 - Science-at-Nanoscale ch05
R
Total potential
energy O V max energy
Potential Secondary minimum
Distance between surfaces
V
A
Primary minimum
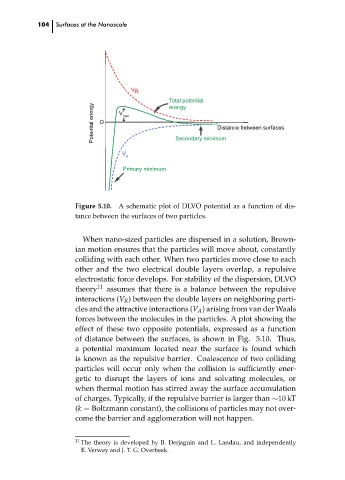
Figure 5.10. A schematic plot of DLVO potential as a function of dis-
tance between the surfaces of two particles.
When nano-sized particles are dispersed in a solution, Brown-
ian motion ensures that the particles will move about, constantly
colliding with each other. When two particles move close to each
other and the two electrical double layers overlap, a repulsive
electrostatic force develops. For stability of the dispersion, DLVO
theory 11 assumes that there is a balance between the repulsive
interactions (V R ) between the double layers on neighboring parti-
cles and the attractive interactions (V A ) arising from van der Waals
forces between the molecules in the particles. A plot showing the
effect of these two opposite potentials, expressed as a function
of distance between the surfaces, is shown in Fig. 5.10. Thus,
a potential maximum located near the surface is found which
is known as the repulsive barrier. Coalescence of two colliding
particles will occur only when the collision is sufficiently ener-
getic to disrupt the layers of ions and solvating molecules, or
when thermal motion has stirred away the surface accumulation
of charges. Typically, if the repulsive barrier is larger than ∼10 kT
(k = Boltzmann constant), the collisions of particles may not over-
come the barrier and agglomeration will not happen.
11 The theory is developed by B. Derjaguin and L. Landau, and independently
E. Verwey and J. T. G. Overbeek.