Page 116 - Science at the nanoscale
P. 116
9:10
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Surfaces at the Nanoscale
106
O
S O O - + ch05
O Na
O AOT
O
O
AOT
Sodium bis(2-ethylhexyl )
sulfosuccinate
W/O
+
+ +
+ +
+ water + O/W 2 Φ
+ pool +
+ +
+ +
+ WATER ISOOCTANE
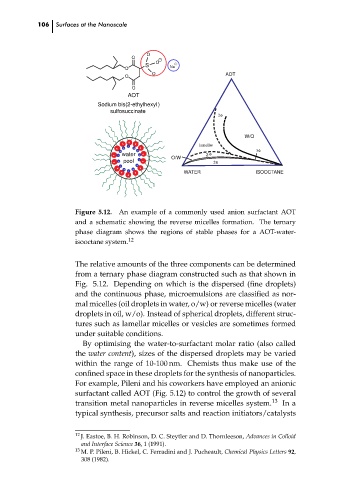
Figure 5.12. An example of a commonly used anion surfactant AOT
and a schematic showing the reverse micelles formation. The ternary
phase diagram shows the regions of stable phases for a AOT-water-
isooctane system. 12
The relative amounts of the three components can be determined
from a ternary phase diagram constructed such as that shown in
Fig. 5.12. Depending on which is the dispersed (fine droplets)
and the continuous phase, microemulsions are classified as nor-
mal micelles (oil droplets in water, o/w) or reverse micelles (water
droplets in oil, w/o). Instead of spherical droplets, different struc-
tures such as lamellar micelles or vesicles are sometimes formed
under suitable conditions.
By optimising the water-to-surfactant molar ratio (also called
the water content), sizes of the dispersed droplets may be varied
within the range of 10-100 nm. Chemists thus make use of the
confined space in these droplets for the synthesis of nanoparticles.
For example, Pileni and his coworkers have employed an anionic
surfactant called AOT (Fig. 5.12) to control the growth of several
transition metal nanoparticles in reverse micelles system. 13 In a
typical synthesis, precursor salts and reaction initiators/catalysts
12 J. Eastoe, B. H. Robinson, D. C. Steytler and D. Thornleeson, Advances in Colloid
and Interface Science 36, 1 (1991).
13 M. P. Pileni, B. Hickel, C. Ferradini and J. Pucheault, Chemical Physics Letters 92,
308 (1982).