Page 115 - Science at the nanoscale
P. 115
RPS: PSP0007 - Science-at-Nanoscale
9:10
June 9, 2009
5.3. Surface stabilisation
While electrostatic stabilisation is very useful for the prepara-
tion of stable colloidal systems, the method is difficult to apply
to multiphase systems (e.g. different solids that carry different 105 ch05
surface charges), and also to electrolyte-sensitive systems. Fur-
thermore, this process is a kinetic stabilization, and it is almost
impossible for the agglomerated particles to be re-dispersed.
Alternatively, steric or electrosteric stabilisation using polymers
or surfactants are applied in most solution preparations of nano-
particles.
5.3.2 Surfactants and Microemulsions
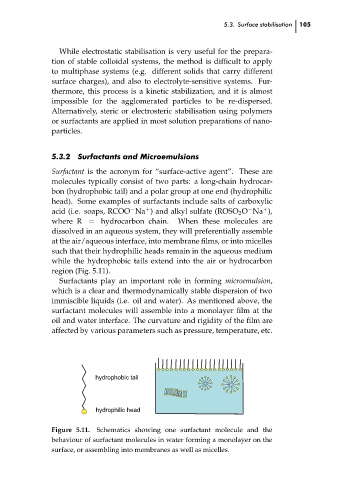
Surfactant is the acronym for “surface-active agent”. These are
molecules typically consist of two parts: a long-chain hydrocar-
bon (hydrophobic tail) and a polar group at one end (hydrophilic
head). Some examples of surfactants include salts of carboxylic
−
−
acid (i.e. soaps, RCOO Na ) and alkyl sulfate (ROSO 2 O Na ),
+
+
where R = hydrocarbon chain. When these molecules are
dissolved in an aqueous system, they will preferentially assemble
at the air/aqueous interface, into membrane films, or into micelles
such that their hydrophilic heads remain in the aqueous medium
while the hydrophobic tails extend into the air or hydrocarbon
region (Fig. 5.11).
Surfactants play an important role in forming microemulsion,
which is a clear and thermodynamically stable dispersion of two
immiscible liquids (i.e. oil and water). As mentioned above, the
surfactant molecules will assemble into a monolayer film at the
oil and water interface. The curvature and rigidity of the film are
affected by various parameters such as pressure, temperature, etc.
hydrophobic tail
hydrophilic head
Figure 5.11. Schematics showing one surfactant molecule and the
behaviour of surfactant molecules in water forming a monolayer on the
surface, or assembling into membranes as well as micelles.