Page 120 - Science at the nanoscale
P. 120
8:11
June 9, 2009
Low-Dimensional Nanostructures
110
to visualise the difference between conductors, insulators and
semiconductors is to plot the available energies for electrons in
the materials. Instead of having discrete energies as in the case of
free atoms (cf. Chapter 3), the available energy states form bands.
An important factor determining electron conduction is whether
or not there are electrons in the conduction band.
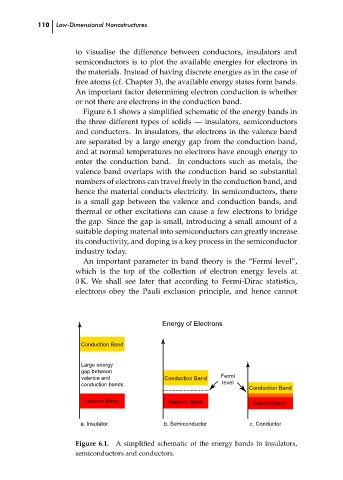
Figure 6.1 shows a simplified schematic of the energy bands in
the three different types of solids — insulators, semiconductors
and conductors. In insulators, the electrons in the valence band
are separated by a large energy gap from the conduction band,
and at normal temperatures no electrons have enough energy to
enter the conduction band. In conductors such as metals, the
valence band overlaps with the conduction band so substantial
numbers of electrons can travel freely in the conduction band, and
hence the material conducts electricity. In semiconductors, there
is a small gap between the valence and conduction bands, and
thermal or other excitations can cause a few electrons to bridge
the gap. Since the gap is small, introducing a small amount of a
suitable doping material into semiconductors can greatly increase
its conductivity, and doping is a key process in the semiconductor
industry today.
An important parameter in band theory is the “Fermi level”,
which is the top of the collection of electron energy levels at
0 K. We shall see later that according to Fermi-Dirac statistics,
electrons obey the Pauli exclusion principle, and hence cannot
Energy of Electrons
Conduction Band RPS: PSP0007 - Science-at-Nanoscale ch06
Large energy
gap between
valence and Conduction Band Fermi
conduction bands. level
Conduction Band
Valence Band Valence Band ValenceBand
a. Insulator b. Semiconductor c. Conductor
Figure 6.1. A simplified schematic of the energy bands in insulators,
semiconductors and conductors.