Page 164 - Science at the nanoscale
P. 164
10:15
RPS: PSP0007 - Science-at-Nanoscale
June 5, 2009
Formation and Self-Assembly at the Nanoscale
154
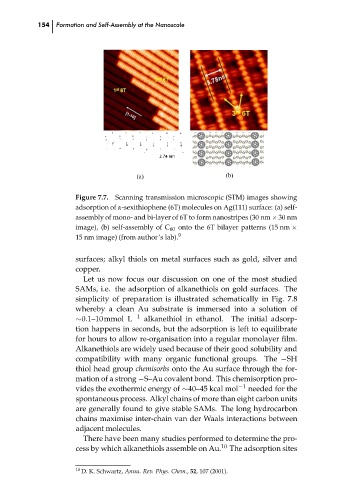
Scanning transmission microscopic (STM) images showing
Figure 7.7.
adsorption of α-sexithiophene (6T) molecules on Ag(111) surface: (a) self-
assembly of mono- and bi-layer of 6T to form nanostripes (30 nm × 30 nm
image), (b) self-assembly of C 60 onto the 6T bilayer patterns (15 nm ×
9
15 nm image) (from author’s lab).
surfaces; alkyl thiols on metal surfaces such as gold, silver and
copper.
Let us now focus our discussion on one of the most studied
SAMs, i.e. the adsorption of alkanethiols on gold surfaces. The
simplicity of preparation is illustrated schematically in Fig. 7.8
whereby a clean Au substrate is immersed into a solution of
−1
alkanethiol in ethanol.
∼0.1–10mmol L
The initial adsorp-
tion happens in seconds, but the adsorption is left to equilibrate
for hours to allow re-organisation into a regular monolayer film. ch07
Alkanethiols are widely used because of their good solubility and
compatibility with many organic functional groups. The −SH
thiol head group chemisorbs onto the Au surface through the for-
mation of a strong −S–Au covalent bond. This chemisorption pro-
vides the exothermic energy of ∼40–45 kcal mol −1 needed for the
spontaneous process. Alkyl chains of more than eight carbon units
are generally found to give stable SAMs. The long hydrocarbon
chains maximise inter-chain van der Waals interactions between
adjacent molecules.
There have been many studies performed to determine the pro-
cess by which alkanethiols assemble on Au. 10 The adsorption sites
10 D. K. Schwartz, Annu. Rev. Phys. Chem., 52, 107 (2001).