Page 165 - Science at the nanoscale
P. 165
RPS: PSP0007 - Science-at-Nanoscale
10:15
June 5, 2009
7.3. The Self-Assembly Processes
Au
Thiol solution
Adsorption
Organisation
Au
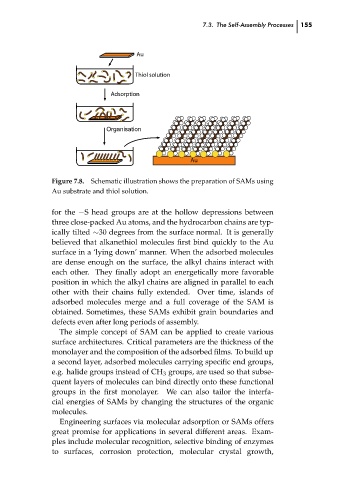
Figure 7.8. Schematic illustration shows the preparation of SAMs using
Au substrate and thiol solution.
for the −S head groups are at the hollow depressions between
three close-packed Au atoms, and the hydrocarbon chains are typ-
ically tilted ∼30 degrees from the surface normal. It is generally
believed that alkanethiol molecules first bind quickly to the Au
surface in a ‘lying down’ manner. When the adsorbed molecules
are dense enough on the surface, the alkyl chains interact with
each other. They finally adopt an energetically more favorable
position in which the alkyl chains are aligned in parallel to each
other with their chains fully extended. Over time, islands of
adsorbed molecules merge and a full coverage of the SAM is
obtained. Sometimes, these SAMs exhibit grain boundaries and
defects even after long periods of assembly.
The simple concept of SAM can be applied to create various
surface architectures. Critical parameters are the thickness of the 155 ch07
monolayer and the composition of the adsorbed films. To build up
a second layer, adsorbed molecules carrying specific end groups,
e.g. halide groups instead of CH 3 groups, are used so that subse-
quent layers of molecules can bind directly onto these functional
groups in the first monolayer. We can also tailor the interfa-
cial energies of SAMs by changing the structures of the organic
molecules.
Engineering surfaces via molecular adsorption or SAMs offers
great promise for applications in several different areas. Exam-
ples include molecular recognition, selective binding of enzymes
to surfaces, corrosion protection, molecular crystal growth,