Page 167 - Science at the nanoscale
P. 167
10:15
June 5, 2009
7.3. The Self-Assembly Processes
deforms as shown schematically in Fig. 7.10. Capillary forces
minimize the Gibbs energies by reducing the interfacial areas.
The amount of capillary interaction between the two particles is
directly proportional to the interfacial deformation created.
extent of deformation is related to the particle size, the surface
energy between air and liquid, the properties of the liquid, etc.
In the immersion situation, deformation strongly depends on the
wetting properties of the two particles and hence the interactions
may be adjusted by adding surfactants to the dispersion. An
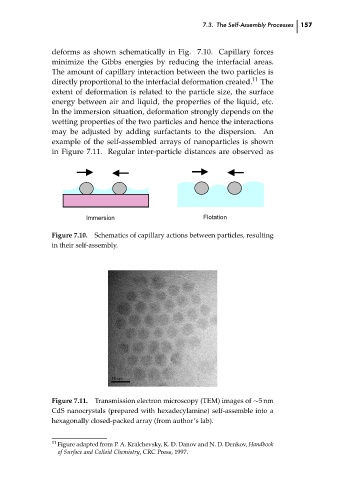
example of the self-assembled arrays of nanoparticles is shown
in Figure 7.11. Regular inter-particle distances are observed as
Flotation
Figure 7.10.
Schematics of capillary actions between particles, resulting
in their self-assembly.
Transmission electron microscopy (TEM) images of ∼5 nm
Figure 7.11. Immersion RPS: PSP0007 - Science-at-Nanoscale 11 The 157 ch07
CdS nanocrystals (prepared with hexadecylamine) self-assemble into a
hexagonally closed-packed array (from author’s lab).
11 Figure adapted from P. A. Kralchevsky, K. D. Danov and N. D. Denkov, Handbook
of Surface and Colloid Chemistry, CRC Press, 1997.