Page 68 - Science at the nanoscale
P. 68
9:2
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Brief Review of Quantum Mechanics
58
one-dimensional potential well, calculate its thickness if the
difference in energy between the first (n = 1) and second
(n = 2) levels is 0.05eV.
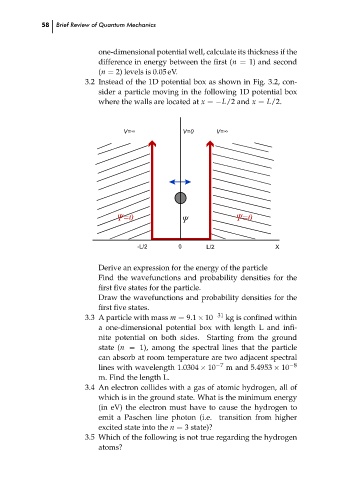
3.2 Instead of the 1D potential box as shown in Fig. 3.2, con-
sider a particle moving in the following 1D potential box
where the walls are located at x = −L/2 and x = L/2.
V=0
Ȍ
Ȍ
Ȍ
-L/2
0
X
Derive an expression for the energy of the particle
Find the wavefunctions and probability densities for the
first five states for the particle.
Draw the wavefunctions and probability densities for the
first five states.
3.3 A particle with mass m = 9.1 × 10
a one-dimensional potential box with length L and infi-
Starting from the ground
nite potential on both sides. L/2 −31 kg is confined within ch03
state (n = 1), among the spectral lines that the particle
can absorb at room temperature are two adjacent spectral
lines with wavelength 1.0304 × 10 −7 m and 5.4953 × 10 −8
m. Find the length L.
3.4 An electron collides with a gas of atomic hydrogen, all of
which is in the ground state. What is the minimum energy
(in eV) the electron must have to cause the hydrogen to
emit a Paschen line photon (i.e. transition from higher
excited state into the n = 3 state)?
3.5 Which of the following is not true regarding the hydrogen
atoms?