Page 63 - Science at the nanoscale
P. 63
9:2
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
3.3. Hydrogen-like Atoms: Orbitals and Atomic Structures
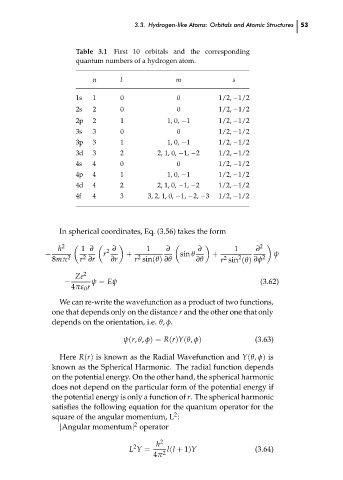
Table 3.1 First 10 orbitals and the corresponding
quantum numbers of a hydrogen atom.
n
l
m
s
1s
0
0
1
1/2, −1/2
2s
0
2
0
1, 0, −1
2
1
2p
1/2, −1/2
1/2, −1/2
0
3s
3
0
1
3p
1/2, −1/2
3
1, 0, −1
2
1/2, −1/2
2, 1, 0, −1, −2
3d
3
1/2, −1/2
4
0
4s
0
1/2, −1/2
1, 0, −1
1
4
4p
2
1/2, −1/2
2, 1, 0, −1, −2
4d
4
4
4f
3
3, 2, 1, 0, −1, −2, −3
1/2, −1/2
In spherical coordinates, Eq. (3.56) takes the form
2
2
1
1
h
1 ∂
∂
∂
∂
∂
2
r
sin θ
−
+
2
2
2
2
2
r ∂r
2
∂r
r sin (θ) ∂φ
8mπ
∂θ
r sin(θ) ∂θ
2
Ze
(3.62)
−
ψ = Eψ
4πε 0 r
We can re-write the wavefunction as a product of two functions,
one that depends only on the distance r and the other one that only
depends on the orientation, i.e. θ, φ.
ψ(r, θ, φ) = R(r)Y(θ, φ) + 1/2, −1/2 (3.63) ψ 53 ch03
Here R(r) is known as the Radial Wavefunction and Y(θ, φ) is
known as the Spherical Harmonic. The radial function depends
on the potential energy. On the other hand, the spherical harmonic
does not depend on the particular form of the potential energy if
the potential energy is only a function of r. The spherical harmonic
satisfies the following equation for the quantum operator for the
2
square of the angular momentum, L :
2
|Angular momentum| operator
h 2
2
L Y = 2 l(l + 1)Y (3.64)
4π