Page 66 - Science at the nanoscale
P. 66
9:2
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Brief Review of Quantum Mechanics
56
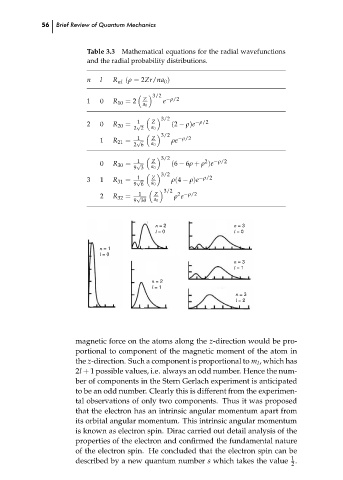
Table 3.3 Mathematical equations for the radial wavefunctions
and the radial probability distributions.
n
l
R
nl
(ρ = 2Zr/na 0 )
3/2
Z
−ρ/2
R 10 = 2
e
0
1
a 0
3/2
1
Z
−ρ/2
2
0
(2 − ρ)e
√
R 20 =
2 2
a 0
3/2
−ρ/2
1
Z
ρe
1
√
R 21 =
2 6
a 0
3/2
2
1
−ρ/2
Z
0
(6 − 6ρ + ρ )e
√
R 30 =
9 3
a 0
3/2
−ρ/2
1
Z
1
ρ(4 − ρ)e
3
√
R 31 =
9 6
a 0
3/2
Z
1
2 −ρ/2
2
ρ e
√
R 32 =
9 30
a 0
n = 3
n = 2
l = 0
l = 0
n = 1
l = 0
n = 3
l = 1
n = 2
l = 1
n = 3
l = 2
magnetic force on the atoms along the z-direction would be pro- ch03
portional to component of the magnetic moment of the atom in
the z-direction. Such a component is proportional to m , which has
l
2l + 1 possible values, i.e. always an odd number. Hence the num-
ber of components in the Stern Gerlach experiment is anticipated
to be an odd number. Clearly this is different from the experimen-
tal observations of only two components. Thus it was proposed
that the electron has an intrinsic angular momentum apart from
its orbital angular momentum. This intrinsic angular momentum
is known as electron spin. Dirac carried out detail analysis of the
properties of the electron and confirmed the fundamental nature
of the electron spin. He concluded that the electron spin can be
1
described by a new quantum number s which takes the value .
2