Page 61 - Science at the nanoscale
P. 61
RPS: PSP0007 - Science-at-Nanoscale
9:2
June 9, 2009
3.3. Hydrogen-like Atoms: Orbitals and Atomic Structures
difference in energy of the two states is released in the form of
radiation, i.e. photons. It is the observations of these emitted pho-
tons with specific wavelengths and hence specific energy values
that provide experimental evidence of the quantisation of energy.
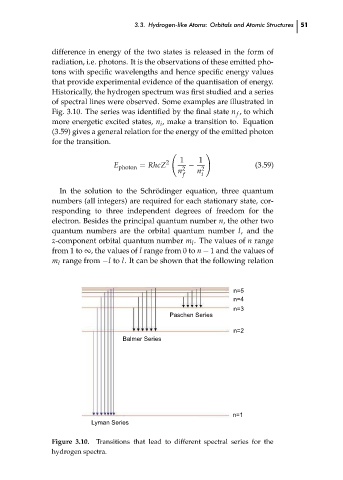
Historically, the hydrogen spectrum was first studied and a series
of spectral lines were observed. Some examples are illustrated in
Fig. 3.10. The series was identified by the final state n , to which
f
more energetic excited states, n , make a transition to. Equation
(3.59) gives a general relation for the energy of the emitted photon
for the transition.
!
1
1
= RhcZ
E
−
photon
2
2
n
n
f
i
In the solution to the Schr¨odinger equation, three quantum
numbers (all integers) are required for each stationary state, cor-
responding to three independent degrees of freedom for the
electron. Besides the principal quantum number n, the other two
quantum numbers are the orbital quantum number l, and the
z-component orbital quantum number m . The values of n range
l
from 1 to ∞, the values of l range from 0 to n − 1 and the values of
m range from −l to l. It can be shown that the following relation
l
n=4
n=3
n=2
Balmer Series i 2 Paschen Series n=5 (3.59) 51 ch03
n=1
Lyman Series
Figure 3.10. Transitions that lead to different spectral series for the
hydrogen spectra.