Page 60 - Science at the nanoscale
P. 60
9:2
RPS: PSP0007 - Science-at-Nanoscale
June 9, 2009
Brief Review of Quantum Mechanics
50
in a hydrogen-like atom:
2
RhcZ
E = −
n
−1
where R is known as the Rydberg constant (= 1.0974 × 10 m
),
and c corresponds to the speed of light. n is the principal quantum
number and its value ranges from 1 to ∞. A common form of the
equation expressed in units of electron volts is given by
(3.58)
2
n
Even though the exact form for the energy differs from the par-
ticle in a potential box, the quantisation of energy is a common
feature of bound systems where the motion of the particle is re-
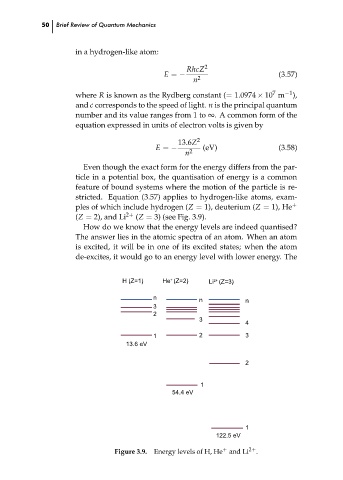
stricted. Equation (3.57) applies to hydrogen-like atoms, exam-
ples of which include hydrogen (Z = 1), deuterium (Z = 1), He
+
(Z = 2), and Li
(Z = 3) (see Fig. 3.9).
2+
How do we know that the energy levels are indeed quantised?
The answer lies in the atomic spectra of an atom. When an atom
is excited, it will be in one of its excited states; when the atom
de-excites, it would go to an energy level with lower energy. The
+
H (Z=1)
He (Z=2)
Li (Z=3)
n
3
2
1 E = − 13.6Z 2 2 n 3 2 (eV) 2+ n 4 3 7 (3.57) ch03
13.6 eV
2
1
54.4 eV
1
122.5 eV
Figure 3.9. Energy levels of H, He and Li 2+ .
+