Page 55 - Science at the nanoscale
P. 55
RPS: PSP0007 - Science-at-Nanoscale
9:2
June 9, 2009
3.2. Basic Postulates of Quantum Mechanics
Case 3: L y , L z ≫ L x = L. In such a case, the quantisation con-
dition (3.29) along both y- and z-directions becomes essentially
continuous. Thus we can write the energy of the particle as
2
2
h
n
2
x
+ k + k
E =
y
2
8m
L
where the quantised band is characterised by n x while k y and
k z are essentially continuous variables. Such a potential system
where the particle is confined by potential wells in one dimension
but free in the other two dimensions is known as a quantum well.
3.2.4
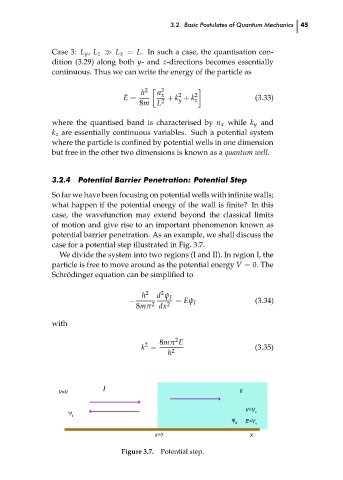
Potential Barrier Penetration: Potential Step
So far we have been focusing on potential wells with infinite walls;
what happen if the potential energy of the wall is finite? In this
case, the wavefunction may extend beyond the classical limits
of motion and give rise to an important phenomenon known as
potential barrier penetration. As an example, we shall discuss the
case for a potential step illustrated in Fig. 3.7.
We divide the system into two regions (I and II). In region I, the
particle is free to move around as the potential energy V = 0. The
Schr¨odinger equation can be simplified to
2
2
h
d ψ I
(3.34)
−
= Eψ I
2
2
8mπ dx
with
2
8mπ E 2 z (3.33) 45 ch03
2
k = 2 (3.35)
h
I
V=0 II
V=V
ψ o
I
ˇ ψ E<V
II o
x=0 X
Figure 3.7. Potential step.