Page 53 - Science at the nanoscale
P. 53
RPS: PSP0007 - Science-at-Nanoscale
9:2
June 9, 2009
3.2. Basic Postulates of Quantum Mechanics
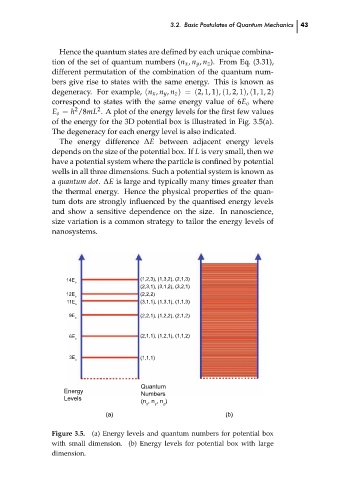
Hence the quantum states are defined by each unique combina-
tion of the set of quantum numbers (n x , n y , n z ). From Eq. (3.31),
different permutation of the combination of the quantum num-
bers give rise to states with the same energy. This is known as
degeneracy. For example, (n x , n y , n z ) = (2, 1, 1), (1, 2, 1), (1, 1, 2)
correspond to states with the same energy value of 6E o where
E o = h /8mL . A plot of the energy levels for the first few values
of the energy for the 3D potential box is illustrated in Fig. 3.5(a).
The degeneracy for each energy level is also indicated.
The energy difference ∆E between adjacent energy levels
depends on the size of the potential box. If L is very small, then we
have a potential system where the particle is confined by potential
wells in all three dimensions. Such a potential system is known as
a quantum dot. ∆E is large and typically many times greater than
the thermal energy. Hence the physical properties of the quan-
tum dots are strongly influenced by the quantised energy levels
and show a sensitive dependence on the size. In nanoscience,
size variation is a common strategy to tailor the energy levels of
nanosystems.
14E
(2,3,1), (3,1,2), (3,2,1)
12E
(2,2,2)
(3,1,1), (1,3,1), (1,1,3)
11E
(2,2,1), (1,2,2), (2,1,2)
9E
(2,1,1), (1,2,1), (1,1,2)
6E 2 o o o o 2 (1,2,3), (1,3,2), (2,1,3) 43 ch03
o
3E (1,1,1)
o
Quantum
Energy Numbers
Levels
(n , n , n )
x y z
(a) (b)
Figure 3.5. (a) Energy levels and quantum numbers for potential box
with small dimension. (b) Energy levels for potential box with large
dimension.