Page 62 - Science at the nanoscale
P. 62
9:2
June 9, 2009
Brief Review of Quantum Mechanics
52
gives the magnitude of the angular momentum, L
h
p
l(l + 1)
L =
2π
and the component of the angular momentum along the
z-direction L z is given by
h
L z = m
(3.61)
l
2π
As with the energy, these quantities are also quantised. The
quantum number l represents the orbital angular momentum of
the electrons, while the quantum number m corresponds to its
l
component along the z-direction.
We have learnt about the energy and angular momentum of the
electron in the hydrogen-like atom. How about the wavefunction?
What about the probability of locating the electron in a certain
region near the nucleus of the atom? This can be determined once
the wavefunctions that satisfy Eq. (3.56) are determined. In addi-
tion, different states of the system are characterised by the set of
quantum numbers (n, l, m ).
l
To solve for the wavefunction, we make use of the fact that
the potential energy (Eq. (3.55)) is spherically symmetric, i.e.
depends only on r. Physical problems where the potential energy
is only a function of the radial distance r are known as central-
force problems. We can simplify the discussion if we re-write
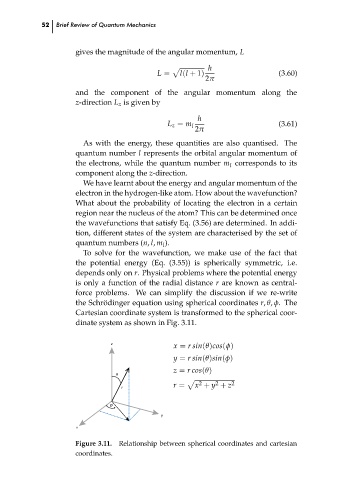
the Schr¨odinger equation using spherical coordinates r, θ, φ. The
Cartesian coordinate system is transformed to the spherical coor-
dinate system as shown in Fig. 3.11.
z RPS: PSP0007 - Science-at-Nanoscale (3.60) ch03
x = r sin(θ)cos(φ)
y = r sin(θ)sin(φ)
z = r cos(θ)
ș
p 2 2 2
x + y + z
r r =
Ø
y
x
Figure 3.11. Relationship between spherical coordinates and cartesian
coordinates.