Page 296 - Separation process engineering
P. 296
azeotrope is heterogeneous; that is, the vapor from the azeotrope will condense to form two liquid phases
that are immiscible. Azeotropic distillation is often performed by adding a solvent or entrainer that forms
an azeotrope with one or both of the components. Before discussing these more complex azeotropic
distillation systems in Section 8.7, let us consider the simpler binary systems that form a heterogeneous
azeotrope.
8.2.1 Binary Heterogeneous Azeotropes
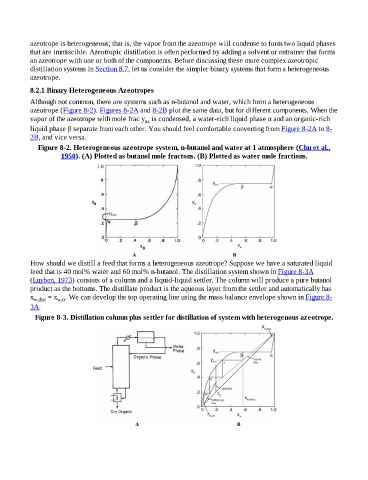
Although not common, there are systems such as n-butanol and water, which form a heterogeneous
azeotrope (Figure 8-2). Figures 8-2A and 8-2B plot the same data, but for different components. When the
vapor of the azeotrope with mole frac y is condensed, a water-rich liquid phase α and an organic-rich
az
liquid phase β separate from each other. You should feel comfortable converting from Figure 8-2A to 8-
2B, and vice versa.
Figure 8-2. Heterogeneous azeotrope system, n-butanol and water at 1 atmosphere (Chu et al.,
1950). (A) Plotted as butanol mole fractons. (B) Plotted as water mole fractions.
How should we distill a feed that forms a heterogeneous azeotrope? Suppose we have a saturated liquid
feed that is 40 mol% water and 60 mol% n-butanol. The distillation system shown in Figure 8-3A
(Luyben, 1973) consists of a column and a liquid-liquid settler. The column will produce a pure butanol
product as the bottoms. The distillate product is the aqueous layer from the settler and automatically has
x w,dist = x w,α . We can develop the top operating line using the mass balance envelope shown in Figure 8-
3A.
Figure 8-3. Distillation column plus settler for distillation of system with heterogenous azeotrope.