Page 163 - Soil and water contamination, 2nd edition
P. 163
150 Soil and Water Contamination
238
232
235
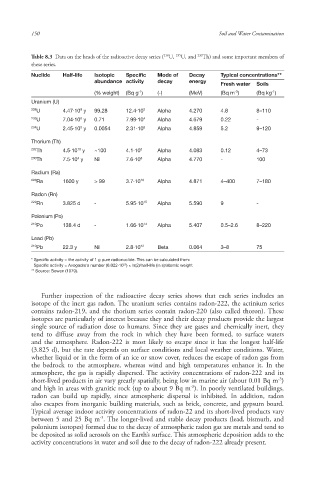
Table 8.3 Data on the heads of the radioactive decay series ( U, U, and Th) and some important members of
these series.
Nuclide Half-life Isotopic Specific Mode of Decay Typical concentrations**
abundance activity decay energy
Fresh water Soils
-1
-3
-1
(% weight) (Bq g ) (-) (MeV) (Bq m ) (Bq kg )
Uranium (U)
238 U 4.47·10 y 99.28 12.4·10 3 Alpha 4.270 4.8 8–110
9
235 8 4
U 7.04·10 y 0.71 7.99·10 Alpha 4.679 0.22 -
234 U 2.45·10 y 0.0054 2.31·10 8 Alpha 4.859 5.2 9–120
5
Thorium (Th)
232 10 3
Th 4.5·10 y ~100 4.1·10 Alpha 4.083 0.12 4–73
230 Th 7.5·10 y Nil 7.6·10 8 Alpha 4.770 - 100
4
Radium (Ra)
226 10
Ra 1600 y > 99 3.7·10 Alpha 4.871 4–400 7–180
Radon (Rn)
222 15
Rn 3.825 d - 5.95·10 Alpha 5.590 9 -
Polonium (Po)
210 Po 138.4 d - 1.66·10 14 Alpha 5.407 0.5–2.6 8–220
Lead (Pb)
210 Pb 22.3 y Nil 2.8·10 12 Beta 0.064 3–8 75
* Specific activity = the activity of 1 g pure radionuclide. This can be calculated from:
23
Specific activity = Avogadro’s number (6.022·10 ) × ln(2)/half-life (in s)/atomic weight
** Source: Bowen (1979).
Further inspection of the radioactive decay series shows that each series includes an
isotope of the inert gas radon . The uranium series contains radon-222, the actinium series
contains radon-219, and the thorium series contain radon-220 (also called thoron). These
isotopes are particularly of interest because they and their decay products provide the largest
single source of radiation dose to humans. Since they are gases and chemically inert, they
tend to diffuse away from the rock in which they have been formed, to surface waters
and the atmosphere. Radon-222 is most likely to escape since it has the longest half-life
(3.825 d), but the rate depends on surface conditions and local weather conditions. Water,
whether liquid or in the form of an ice or snow cover, reduces the escape of radon gas from
the bedrock to the atmosphere, whereas wind and high temperatures enhance it. In the
atmosphere, the gas is rapidly dispersed. The activity concentrations of radon-222 and its
-3
short-lived products in air vary greatly spatially, being low in marine air (about 0.01 Bq m )
-3
and high in areas with granitic rock (up to about 9 Bq m ). In poorly ventilated buildings,
radon can build up rapidly, since atmospheric dispersal is inhibited. In addition, radon
also escapes from inorganic building materials, such as brick, concrete, and gypsum board.
Typical average indoor activity concentrations of radon-22 and its short-lived products vary
-3
between 5 and 25 Bq m . The longer-lived and stable decay products (lead , bismuth, and
polonium isotopes) formed due to the decay of atmospheric radon gas are metals and tend to
be deposited as solid aerosols on the Earth’s surface. This atmospheric deposition adds to the
activity concentrations in water and soil due to the decay of radon-222 already present.
10/1/2013 6:44:37 PM
Soil and Water.indd 162
Soil and Water.indd 162 10/1/2013 6:44:37 PM