Page 164 - Soil and water contamination, 2nd edition
P. 164
Radionuclides 151
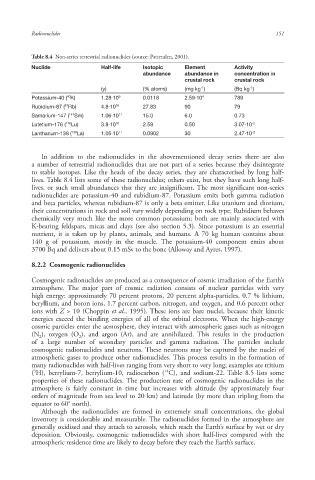
Table 8.4 Non-series terrestrial radionuclides (source: Pattenden, 2001).
Nuclide Half-life Isotopic Element Activity
abundance abundance in concentration in
crustal rock crustal rock
-1
-1
(y) (% atoms) (mg kg ) (Bq kg )
40
Potassium-40 ( K) 1.28·10 9 0.0118 2.59·10 4 789
Rubidium-87 ( Rb) 4.8·10 10 27.83 90 79
87
Samarium-147 ( 147 Sm) 1.06·10 11 15.0 6.0 0.73
-2
Lutetium-176 ( 176 Lu) 3.8·10 10 2.59 0.50 3.07·10
Lanthanum-138 ( 138 La) 1.05·10 11 0.0902 30 2.47·10 -2
In addition to the radionuclides in the abovementioned decay series there are also
a number of terrestrial radionuclides that are not part of a series because they disintegrate
to stable isotopes. Like the heads of the decay series, they are characterised by long half-
lives. Table 8.4 lists some of these radionuclides; others exist, but they have such long half-
lives, or such small abundances that they are insignificant. The most significant non-series
radionuclides are potassium -40 and rubidium-87. Potassium emits both gamma radiation
and beta particles, whereas rubidium-87 is only a beta emitter. Like uranium and thorium ,
their concentrations in rock and soil vary widely depending on rock type. Rubidium behaves
chemically very much like the more common potassium; both are mainly associated with
K-bearing feldspars , micas and clays (see also section 5.3). Since potassium is an essential
nutrient, it is taken up by plants, animals, and humans. A 70 kg human contains about
140 g of potassium, mostly in the muscle. The potassium-40 component emits about
3700 Bq and delivers about 0.15 mSv to the bone (Alloway and Ayres, 1997).
8.2.2 Cosmogenic radionuclides
Cosmogenic radionuclides are produced as a consequence of cosmic irradiation of the Earth’s
atmosphere. The major part of cosmic radiation consists of nuclear particles with very
high energy: approximately 70 percent protons , 20 percent alpha-particles, 0.7 % lithium,
beryllium, and boron ions, 1.7 percent carbon, nitrogen , and oxygen, and 0.6 percent other
ions with Z > 10 (Choppin et al., 1995). These ions are bare nuclei, because their kinetic
energies exceed the binding energies of all of the orbital electrons. When the high-energy
cosmic particles enter the atmosphere, they interact with atmospheric gases such as nitrogen
(N ), oxygen (O ), and argon (Ar), and are annihilated. This results in the production
2 2
of a large number of secondary particles and gamma radiation . The particles include
cosmogenic radionuclides and neutrons. These neutrons may be captured by the nuclei of
atmospheric gases to produce other radionuclides. This process results in the formation of
many radionuclides with half-lives ranging from very short to very long; examples are tritium
14
3
( H), berrylium-7, berrylium-10, radiocarbon ( C), and sodium -22. Table 8.5 lists some
properties of these radionuclides. The production rate of cosmogenic radionuclides in the
atmosphere is fairly constant in time but increases with altitude (by approximately four
orders of magnitude from sea level to 20 km) and latitude (by more than tripling from the
equator to 60° north).
Although the radionuclides are formed in extremely small concentrations, the global
inventory is considerable and measurable. The radionuclides formed in the atmosphere are
generally oxidised and they attach to aerosols , which reach the Earth’s surface by wet or dry
deposition . Obviously, cosmogenic radionuclides with short half-lives compared with the
atmospheric residence time are likely to decay before they reach the Earth’s surface.
10/1/2013 6:44:37 PM
Soil and Water.indd 163 10/1/2013 6:44:37 PM
Soil and Water.indd 163