Page 165 - Soil and water contamination, 2nd edition
P. 165
152 Soil and Water Contamination
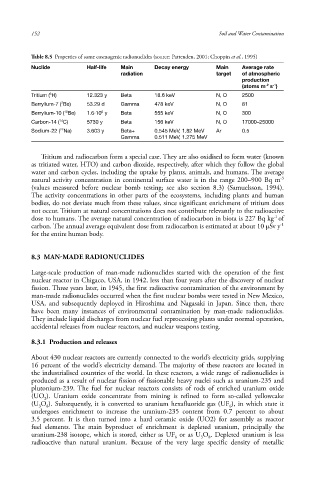
Table 8.5 Properties of some cosmogenic radionuclides (source: Pattenden, 2001; Choppin et al., 1995)
Nuclide Half-life Main Decay energy Main Average rate
radiation target of atmospheric
production
-2
-1
(atoms m s )
3
Tritium ( H) 12.323 y Beta 18.6 keV N, O 2500
7
Berrylium-7 ( Be) 53.29 d Gamma 478 keV N, O 81
6
Berrylium-10 ( Be) 1.6·10 y Beta 555 keV N, O 300
10
14
Carbon-14 ( C) 5730 y Beta 156 keV N, O 17000–25000
22
Sodium-22 ( Na) 3.603 y Beta+ 0.545 MeV, 1.82 MeV Ar 0.5
Gamma 0.511 MeV, 1.275 MeV
Tritium and radiocarbon form a special case. They are also oxidised to form water (known
as tritiated water, HTO) and carbon dioxide , respectively, after which they follow the global
water and carbon cycles, including the uptake by plants, animals, and humans. The average
-3
natural activity concentration in continental surface water is in the range 200–900 Bq m
(values measured before nuclear bomb testing; see also section 8.3) (Samuelsson, 1994).
The activity concentrations in other parts of the ecosystems, including plants and human
bodies, do not deviate much from these values, since significant enrichment of tritium does
not occur. Tritium at natural concentrations does not contribute relevantly to the radioactive
-1
dose to humans. The average natural concentration of radiocarbon in biota is 227 Bq kg of
-1
carbon. The annual average equivalent dose from radiocarbon is estimated at about 10 μSv y
for the entire human body.
8.3 MAN-MADE RADIONUCLIDES
Large-scale production of man-made radionuclides started with the operation of the first
nuclear reactor in Chigaco, USA, in 1942, less than four years after the discovery of nuclear
fission . Three years later, in 1945, the first radioactive contamination of the environment by
man-made radionuclides occurred when the first nuclear bombs were tested in New Mexico,
USA, and subsequently deployed in Hiroshima and Nagasaki in Japan. Since then, there
have been many instances of environmental contamination by man-made radionuclides.
They include liquid discharges from nuclear fuel reprocessing plants under normal operation,
accidental releases from nuclear reactors, and nuclear weapons testing.
8.3.1 Production and releases
About 430 nuclear reactors are currently connected to the world’s electricity grids, supplying
16 percent of the world’s electricity demand. The majority of these reactors are located in
the industrialised countries of the world. In these reactors, a wide range of radionuclides is
produced as a result of nuclear fission of fissionable heavy nuclei such as uranium -235 and
plutonium -239. The fuel for nuclear reactors consists of rods of enriched uranium oxide
(UO ). Uranium oxide concentrate from mining is refined to form so-called yellowcake
2
(U O ). Subsequently, it is converted to uranium hexafluoride gas (UF ), in which state it
3 8 6
undergoes enrichment to increase the uranium-235 content from 0.7 percent to about
3.5 percent. It is then turned into a hard ceramic oxide (UO2) for assembly as reactor
fuel elements. The main byproduct of enrichment is depleted uranium , principally the
uranium-238 isotope, which is stored, either as UF or as U O . Depleted uranium is less
6 3 8
radioactive than natural uranium. Because of the very large specific density of metallic
10/3/2013 2:37:57 PM
Soil and Water.indd 164 10/3/2013 2:37:57 PM
Soil and Water.indd 164