Page 161 - Soil and water contamination, 2nd edition
P. 161
148 Soil and Water Contamination
Z=80 81 82 83 84 85 86 87 88 89 90 91 92
Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U
234 238
Uranium-Radium A=4n+2
24.1 d 4.5 10 9 y
234
6.7 h
214 218 222 226 230 234
26.8 m 3.05 m 3.382 d 1600 y 7.5 10 4 y 2.5 10 5 y
214
19.9 m
210 214
22.3 y 164 µs
210 A
a
5.0 y half-life
206 210
b
stable 138.4 d
231 235
Actinium A=4n+3
25.5 h 7.04 10 8 y
227 231
21.77 y 3.28 10 4 y
211 215 219 223 227
36.1 m 1.78 s 3.96 s 11.43 d 18.72 d
207 211
4.77 m 2.17 m
207
stable
228 232
Thorium A=4n
5.75 d 1.4 10 10 y
228
6.13 h
212 216 220 224 228
10.64 h 0.15 s 55.6 s 3.66 d 1.913 y
208 212
3.053 m 60.6 m
208 212
stable 0.3 µs
Z=80 81 82 83 84 85 86 87 88 89 90 91 92
Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U
6955
6 6955 5
9
5
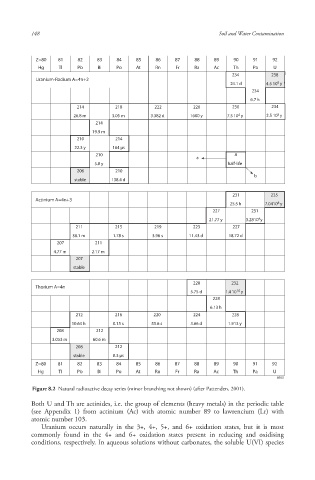
Figure 8.2 Natural radioactive decay series (minor branching not shown) (after Pattenden, 2001).
Both U and Th are actinides, i.e. the group of elements (heavy metals) in the periodic table
(see Appendix 1) from actinium (Ac) with atomic number 89 to lawrencium (Lr) with
atomic number 103.
Uranium occurs naturally in the 3+, 4+, 5+, and 6+ oxidation state s, but it is most
commonly found in the 4+ and 6+ oxidation states present in reducing and oxidising
conditions, respectively. In aqueous solutions without carbonates, the soluble U(VI) species
10/1/2013 6:44:35 PM
Soil and Water.indd 160 10/1/2013 6:44:35 PM
Soil and Water.indd 160