Page 326 - Soil and water contamination, 2nd edition
P. 326
Patterns in groundwater 313
Recharge
Discharge
Acid
Temp
Polluted
(Sub) oxic
Pressure admixing
decrease
Basic
Acid exchange
Increased
Anoxic
Unpolluted
+ pressure increase
Strong quality fluctuations
Equillibrium
Methanogenic
Base-exchange
6642 6642 6642
Constant quality
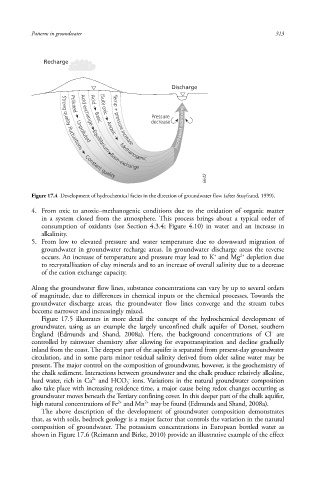
Figure 17.4 Development of hydrochemical facies in the direction of groundwater flow (after Stuyfzand, 1999).
4. From oxic to anoxic –methanogenic conditions due to the oxidation of organic matter
in a system closed from the atmosphere. This process brings about a typical order of
consumption of oxidants (see Section 4.3.4; Figure 4.10) in water and an increase in
alkalinity .
5. From low to elevated pressure and water temperature due to downward migration of
groundwater in groundwater recharge areas. In groundwater discharge areas the reverse
2+
+
occurs. An increase of temperature and pressure may lead to K and Mg depletion due
to recrystallisation of clay minerals and to an increase of overall salinity due to a decrease
of the cation exchange capacity .
Along the groundwater flow lines, substance concentrations can vary by up to several orders
of magnitude, due to differences in chemical inputs or the chemical processes. Towards the
groundwater discharge areas, the groundwater flow lines converge and the stream tube s
become narrower and increasingly mixed.
Figure 17.5 illustrates in more detail the concept of the hydrochemical development of
groundwater, using as an example the largely unconfined chalk aquifer of Dorset, southern
-
England (Edmunds and Shand, 2008a). Here, the background concentrations of Cl are
controlled by rainwater chemistry after allowing for evapotranspiration and decline gradually
inland from the coast. The deepest part of the aquifer is separated from present-day groundwater
circulation, and in some parts minor residual salinity derived from older saline water may be
present. The major control on the composition of groundwater, however, is the geochemistry of
the chalk sediment. Interactions between groundwater and the chalk produce relatively alkaline,
-
2+
hard water, rich in Ca and HCO ions. Variations in the natural groundwater composition
3
also take place with increasing residence time, a major cause being redox changes occurring as
groundwater moves beneath the Tertiary confining cover. In this deeper part of the chalk aquifer,
2+
2+
high natural concentrations of Fe and Mn may be found (Edmunds and Shand, 2008a).
The above description of the development of groundwater composition demonstrates
that, as with soils, bedrock geology is a major factor that controls the variation in the natural
composition of groundwater. The potassium concentrations in European bottled water as
shown in Figure 17.6 (Reimann and Birke, 2010) provide an illustrative example of the effect
10/3/2013 2:37:58 PM
Soil and Water.indd 325
Soil and Water.indd 325 10/3/2013 2:37:58 PM