Page 340 - Soil and water contamination, 2nd edition
P. 340
Patterns in groundwater 327
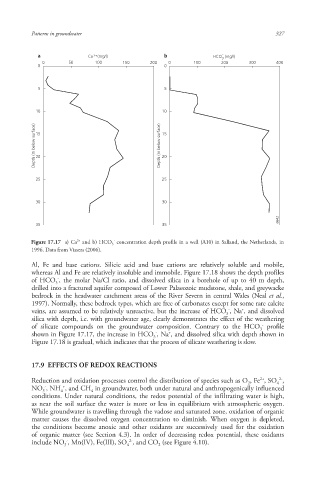
a Ca 2+ (mg/l) b HCO - 3 (mg/l)
0 50 100 150 200 0 100 200 300 400
0 0
5 5
10 10
Depth (m below surface) 15 Depth (m below surface) 15
20
20
25 25
30 30
6642
35 35
2+
-
Figure 17.17 a) Ca and b) HCO 3 concentration depth profile in a well (A10) in Salland, the Netherlands, in
1996. Data from Vissers (2006).
Al, Fe and base cations. Silicic acid and base cations are relatively soluble and mobile,
whereas Al and Fe are relatively insoluble and immobile. Figure 17.18 shows the depth profiles
-
of HCO , the molar Na/Cl ratio, and dissolved silica in a borehole of up to 40 m depth,
3
drilled into a fractured aquifer composed of Lower Palaeozoic mudstone, shale, and greywacke
bedrock in the headwater catchment areas of the River Severn in central Wales (Neal et al.,
1997). Normally, these bedrock types, which are free of carbonates except for some rare calcite
-
+
veins, are assumed to be relatively unreactive, but the increase of HCO , Na , and dissolved
3
silica with depth, i.e. with groundwater age, clearly demonstrates the effect of the weathering
-
of silicate compounds on the groundwater composition. Contrary to the HCO profile
3
+
-
shown in Figure 17.17, the increase in HCO , Na , and dissolved silica with depth shown in
3
Figure 17.18 is gradual, which indicates that the process of silicate weathering is slow.
17.9 EFFECTS OF REDOX REACTIONS
2-
2+
Reduction and oxidation processes control the distribution of species such as O , Fe , SO ,
4
2
+
-
NO , NH , and CH in groundwater, both under natural and anthropogenically influenced
3 4 4
conditions. Under natural conditions, the redox potential of the infiltrating water is high,
as near the soil surface the water is more or less in equilibrium with atmospheric oxygen .
While groundwater is travelling through the vadose and saturated zone, oxidation of organic
matter causes the dissolved oxygen concentration to diminish. When oxygen is depleted,
the conditions become anoxic and other oxidants are successively used for the oxidation
of organic matter (see Section 4.3). In order of decreasing redox potential, these oxidants
-
2-
include NO , Mn(IV) , Fe(III) , SO , and CO (see Figure 4.10).
3 4 2
10/1/2013 6:47:07 PM
Soil and Water.indd 339
Soil and Water.indd 339 10/1/2013 6:47:07 PM