Page 341 - Soil and water contamination, 2nd edition
P. 341
328 Soil and Water Contamination
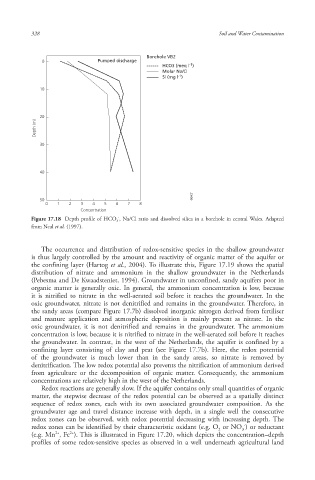
Borehole VB2
0 Pumped discharge
HCO3 (meq l -1 )
Molar Na/Cl
Sl (mg l -1 )
10
20
Depth (m)
30
40
6642 6642 6642
50
0 1 2 3 4 5 6 7 8
Concentration
-
Figure 17.18 Depth profile of HCO 3 , Na/Cl ratio and dissolved silica in a borehole in central Wales . Adapted
from Neal et al. (1997).
The occurrence and distribution of redox-sensitive species in the shallow groundwater
is thus largely controlled by the amount and reactivity of organic matter of the aquifer or
the confining layer (Hartog et al., 2004). To illustrate this, Figure 17.19 shows the spatial
distribution of nitrate and ammonium in the shallow groundwater in the Netherlands
(Pebesma and De Kwaadsteniet, 1994). Groundwater in unconfined , sandy aquifers poor in
organic matter is generally oxic . In general, the ammonium concentration is low, because
it is nitrified to nitrate in the well-aerated soil before it reaches the groundwater. In the
oxic groundwater, nitrate is not denitrified and remains in the groundwater. Therefore, in
the sandy areas (compare Figure 17.7b) dissolved inorganic nitrogen derived from fertiliser
and manure application and atmospheric deposition is mainly present as nitrate. In the
oxic groundwater, it is not denitrified and remains in the groundwater. The ammonium
concentration is low, because it is nitrified to nitrate in the well-aerated soil before it reaches
the groundwater. In contrast, in the west of the Netherlands, the aquifer is confined by a
confining layer consisting of clay and peat (see Figure 17.7b). Here, the redox potential
of the groundwater is much lower than in the sandy areas, so nitrate is removed by
denitrification . The low redox potential also prevents the nitrification of ammonium derived
from agriculture or the decomposition of organic matter. Consequently, the ammonium
concentrations are relatively high in the west of the Netherlands.
Redox reactions are generally slow. If the aquifer contains only small quantities of organic
matter, the stepwise decrease of the redox potential can be observed as a spatially distinct
sequence of redox zones , each with its own associated groundwater composition. As the
groundwater age and travel distance increase with depth, in a single well the consecutive
redox zones can be observed, with redox potential decreasing with increasing depth. The
-
redox zones can be identified by their characteristic oxidant (e.g. O or NO ) or reductant
2
3
2+
2+
(e.g. Mn , Fe ). This is illustrated in Figure 17.20, which depicts the concentration–depth
profiles of some redox-sensitive species as observed in a well underneath agricultural land
10/1/2013 6:47:07 PM
Soil and Water.indd 340 10/1/2013 6:47:07 PM
Soil and Water.indd 340