Page 360 - Soil and water contamination, 2nd edition
P. 360
Patterns in surface water 347
a 120 b 120
6955
100
100
Concentration (mg l -1 )/ Discharge (m 3 s -1 ) 80 Concentration (mg l -1 ) 80
60
60
40
40
20
0 20 0
50 100 150 200 250 10 20 40 60 80
Time (h)
Discharge (m 3 /s)
c 120 d 120
100
100
Concentration (mg l -1 )/ Discharge (m 3 s -1 ) 80 Concentration (mg l -1 ) 80
60
60
40
40
20
0 20 0
50 100 150 200 250 10 20 40 60 80
Time (h) Discharge (m 3 /s)
e 120 f 120
100
100
Concentration (mg l -1 )/ Discharge (m 3 s -1 ) 80 Concentration (mg l -1 ) 80
60
60
40
40
20
0 20 0
50 100 150 200 250 10 20 40 60 80
Time (h) Discharge (m 3 /s)
g 120 h 120
100
100
Concentration (mg l -1 )/ Discharge (m 3 s -1 ) 80 Concentration (mg l -1 ) 80
60
60
40
40
20
0 20 0
50 100 150 200 250 10 20 40 60 80
Time (h) Discharge (m 3 /s)
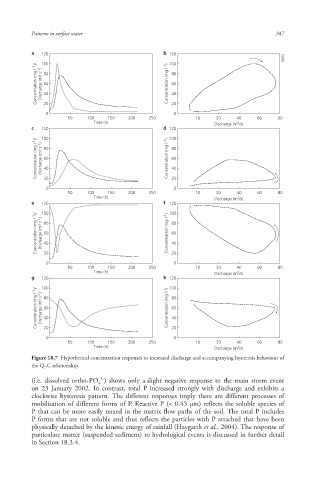
Figure 18.7 Hypothetical concentration responses to increased discharge and accompanying hysteresis behaviour of
the Q–C relationship.
3-
(i.e. dissolved ortho-PO ) shows only a slight negative response to the main storm event
4
on 23 January 2002. In contrast, total P increased strongly with discharge and exhibits a
clockwise hysteresis pattern. The different responses imply there are different processes of
mobilisation of different forms of P. Reactive P (< 0.45 μm) reflects the soluble species of
P that can be more easily mixed in the matrix flow paths of the soil. The total P includes
P forms that are not soluble and thus reflects the particles with P attached that have been
physically detached by the kinetic energy of rainfall (Haygarth et al., 2004). The response of
particulate matter (suspended sediment ) to hydrological events is discussed in further detail
in Section 18.3.4.
10/1/2013 6:47:12 PM
Soil and Water.indd 359 10/1/2013 6:47:12 PM
Soil and Water.indd 359