Page 24 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 24
12 Reservoir Engineering
-
"I
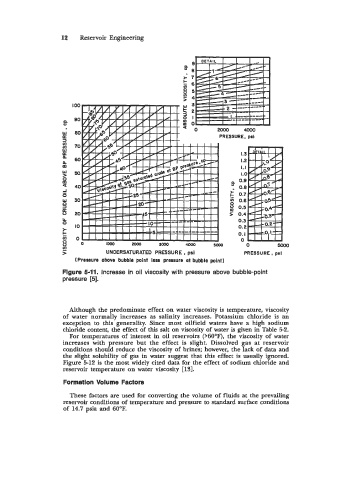
> UNDERSATURATED PRESSURE , psi PRESSURE, psi
(Pressure above bubble point less pressure ot bubble point)
Figure 5-11. Increase in oil viscosity with pressure above bubble-point
pressure [5].
Although the predominate effect on water viscosity is temperature, viscosity
of water normally increases as salinity increases. Potassium chloride is an
exception to this generality. Since most oilfield waters have a high sodium
chloride content, the effect of this salt on viscosity of water is given in Table 5-2.
For temperatures of interest in oil reservoirs (>60°F), the viscosity of water
increases with pressure but the effect is slight. Dissolved gas at reservoir
conditions should reduce the viscosity of brines; however, the lack of data and
the slight solubility of gas in water suggest that this effect is usually ignored.
Figure 5-12 is the most widely cited data for the effect of sodium chloride and
reservoir temperature on water viscosity [ 131.
Formatlon Volume Factors
These factors are used for converting the volume of fluids at the prevailing
reservoir conditions of temperature and pressure to standard surface conditions
of 14.7 psia and 6OOF.