Page 25 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 25
Basic Principles, Definitions, and Data 11
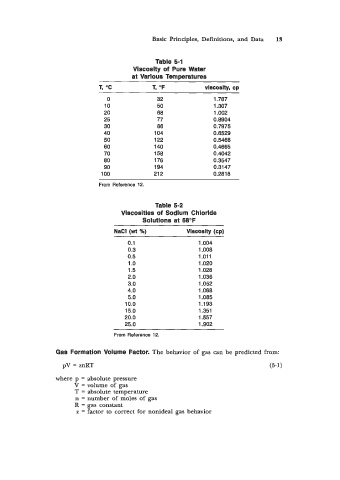
Table 5-1
Viscosity of Pure Water
at Varlous Temperatures
1, "C 1, "F vlscOsltyl cp
0 32 1.787
10 50 1.307
20 68 1.002
25 77 0.8904
30 86 0.7975
40 104 0.6529
50 1 22 0.5468
60 140 0.4665
70 158 0.4042
80 176 0.3547
90 194 0.3147
100 21 2 0.2818
From Reference 12.
Table 5-2
Vlscosities of Sodium Chlorlde
Solutions at 68°F
NaCl (wt X) Vlscoslty (cp)
0.1 1.004
0.3 1.008
0.5 1.011
1 .o 1.020
1.5 1.028
2.0 1.036
3.0 1.052
4.0 1.068
5.0 1.085
10.0 1.193
15.0 1.351
20.0 1.557
25.0 1.902
From Reference 12.
Gas Formation Volume Factor. The behavior of gas can be predicted from:
pV = znRT (5-1)
where p = absolute pressure
V = volume of gas
T = absolute temperature
n = number of moles of gas
R = gas constant
z = factor to correct for nonideal gas behavior