Page 406 - Standard Handbook Petroleum Natural Gas Engineering VOLUME2
P. 406
872 Production
Single phose
90s
B
*C
Reservoir temperature -c
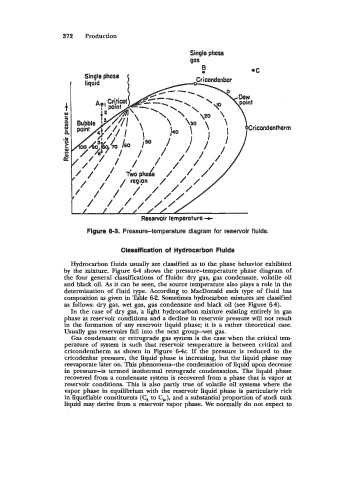
Figure 0-3. Pressure-temperature diagram for reservoir fluids.
Classiflcatlon of Hydrocarbon Fluids
Hydrocarbon fluids usually are classified as to the phase behavior exhibited
by the mixture. Figure 6-4 shows the pressure-temperature phase diagram of
the four general classifications of fluids: dry gas, gas condensate, volatile oil
and black oil. As it can be seen, the source temperature also plays a role in the
determination of fluid type. According to MacDonald each type of fluid has
composition as given in Table 6-2. Sometimes hydrocarbon mixtures are classified
as follows: dry gas, wet gas, gas condensate and black oil (see Figure 64).
In the case of dry gas, a light hydrocarbon mixture existing entirely in gas
phase at reservoir conditions and a decline in reservoir pressure will not result
in the formation of any reservoir liquid phase; it is a rather theoretical case.
Usually gas reservoirs fall into the next group-wet gas.
Gas condensate or retrograde gas system is the case when the critical tem-
perature of system is such that reservoir temperature is between critical and
cricondentherm as shown in Figure 64. If the pressure is reduced to the
cricodenbar pressure, the liquid phase is increasing, but the liquid phase may
reevaporate later on. This phenomena-the condensation of liquid upon decrease
in pressure-is termed isothermal retrograde condensation. The liquid phase
recovered from a condensate system is recovered from a phase that is vapor at
reservoir conditions. This is also partly true of volatile oil systems where the
vapor phase in equilibrium with the reservoir liquid phase is particularly rich
in liquefiable constituents (C, to C$, and a substantial proportion of stock tank
liquid may derive from a reservoir vapor phase. We normally do not expect to