Page 53 - Synthetic Fuels Handbook
P. 53
NATURAL GAS 41
At the boiling point, a substance changes its state from liquid to gas. A stricter definition
of boiling point is the temperature at which the liquid and vapor (gas) phases of a substance
can exist in equilibrium. When heat is applied to a liquid, the temperature of the liquid rises
until the vapor pressure of the liquid equals the pressure of the surrounding atmosphere
(gases). At this point there is no further rise in temperature, and the additional heat energy
supplied is absorbed as latent heat of vaporization to transform the liquid into gas. This
transformation occurs not only at the surface of the liquid (as in the case of evaporation) but
also throughout the volume of the liquid, where bubbles of gas are formed. The boiling point
of a liquid is lowered if the pressure of the surrounding atmosphere (gases) is decreased. On
the other hand, if the pressure of the surrounding atmosphere (gases) is increased, the boil-
ing point is raised. For this reason, it is customary when the boiling point of a substance is
given to include the pressure at which it is observed, if that pressure is other than standard,
that is, 760 mm Hg or 1 atm (STP, Standard Temperature and Pressure).
The boiling points of petroleum fractions are rarely, if ever, distinct temperatures. It is,
in fact, more correct to refer to the boiling ranges of the various fractions; the same is true
of natural gas. To determine these ranges, the material in question is tested in various meth-
ods of distillation, either at atmospheric pressure or at reduced pressure. Thus, the boiling
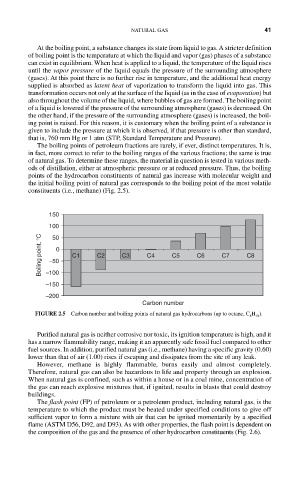
points of the hydrocarbon constituents of natural gas increase with molecular weight and
the initial boiling point of natural gas corresponds to the boiling point of the most volatile
constituents (i.e., methane) (Fig. 2.5).
150
100
Boiling point, °C –50 0 C1 C2 C3 C4 C5 C6 C7 C8
50
–100
–150
–200
Carbon number
FIGURE 2.5 Carbon number and boiling points of natural gas hydrocarbons (up to octane, C 8 H 18 ).
Purified natural gas is neither corrosive nor toxic, its ignition temperature is high, and it
has a narrow flammability range, making it an apparently safe fossil fuel compared to other
fuel sources. In addition, purified natural gas (i.e., methane) having a specific gravity (0.60)
lower than that of air (1.00) rises if escaping and dissipates from the site of any leak.
However, methane is highly flammable, burns easily and almost completely.
Therefore, natural gas can also be hazardous to life and property through an explosion.
When natural gas is confined, such as within a house or in a coal mine, concentration of
the gas can reach explosive mixtures that, if ignited, results in blasts that could destroy
buildings.
The flash point (FP) of petroleum or a petroleum product, including natural gas, is the
temperature to which the product must be heated under specified conditions to give off
sufficient vapor to form a mixture with air that can be ignited momentarily by a specified
flame (ASTM D56, D92, and D93). As with other properties, the flash point is dependent on
the composition of the gas and the presence of other hydrocarbon constituents (Fig. 2.6).