Page 55 - Synthetic Fuels Handbook
P. 55
NATURAL GAS 43
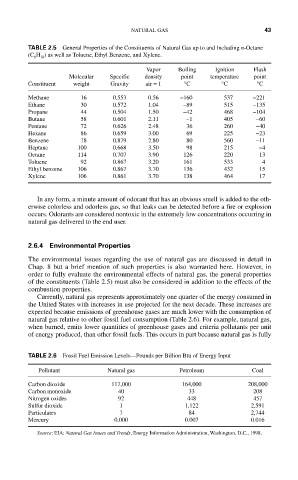
TABLE 2.5 General Properties of the Constituents of Natural Gas up to and Including n-Octane
(C H ) as well as Toluene, Ethyl Benzene, and Xylene.
8
18
Vapor Boiling Ignition Flash
Molecular Specific density point temperature point
Constituent weight Gravity air = 1 °C °C °C
Methane 16 0.553 0.56 −160 537 −221
Ethane 30 0.572 1.04 −89 515 −135
Propane 44 0.504 1.50 −42 468 −104
Butane 58 0.601 2.11 −1 405 −60
Pentane 72 0.626 2.48 36 260 −40
Hexane 86 0.659 3.00 69 225 −23
Benzene 78 0.879 2.80 80 560 −11
Heptane 100 0.668 3.50 98 215 −4
Octane 114 0.707 3.90 126 220 13
Toluene 92 0.867 3.20 161 533 4
Ethyl benzene 106 0.867 3.70 136 432 15
Xylene 106 0.861 3.70 138 464 17
In any form, a minute amount of odorant that has an obvious smell is added to the oth-
erwise colorless and odorless gas, so that leaks can be detected before a fire or explosion
occurs. Odorants are considered nontoxic in the extremely low concentrations occurring in
natural gas delivered to the end user.
2.6.4 Environmental Properties
The environmental issues regarding the use of natural gas are discussed in detail in
Chap. 8 but a brief mention of such properties is also warranted here. However, in
order to fully evaluate the environmental effects of natural gas, the general properties
of the constituents (Table 2.5) must also be considered in addition to the effects of the
combustion properties.
Currently, natural gas represents approximately one quarter of the energy consumed in
the United States with increases in use projected for the next decade. These increases are
expected because emissions of greenhouse gases are much lower with the consumption of
natural gas relative to other fossil fuel consumption (Table 2.6). For example, natural gas,
when burned, emits lower quantities of greenhouse gases and criteria pollutants per unit
of energy produced, than other fossil fuels. This occurs in part because natural gas is fully
TABLE 2.6 Fossil Fuel Emission Levels—Pounds per Billion Btu of Energy Input
Pollutant Natural gas Petroleum Coal
Carbon dioxide 117,000 164,000 208,000
Carbon monoxide 40 33 208
Nitrogen oxides 92 448 457
Sulfur dioxide 1 1,122 2,591
Particulates 7 84 2,744
Mercury 0.000 0.007 0.016
Source: EIA: Natural Gas Issues and Trends, Energy Information Administration, Washington, D.C., 1998.