Page 60 - Synthetic Fuels Handbook
P. 60
48 CHAPTER TWO
The principal benefits of physical solvents are: (a) high selectivity for hydrogen
sulfide over carbonyl sulfide and carbon dioxide, (b) high loadings at high acid gas
partial pressures, (c) solvent stability, and (d) low heat requirements because most of the
solvent can be regenerated by a simple pressure letdown.
The performance of a physical solvent can be easily predicted. The solubility of a com-
pound in the solvent is directly proportional to its partial pressure in the gas phase, hence,
the improvement in the performance of physical solvent processes with increasing gas
pressure.
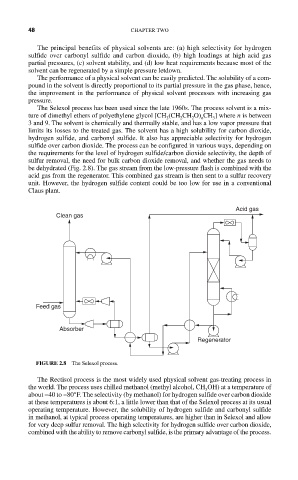
The Selexol process has been used since the late 1960s. The process solvent is a mix-
ture of dimethyl ethers of polyethylene glycol [CH (CH CH O) CH ] where n is between
n
3
2
2
3
3 and 9. The solvent is chemically and thermally stable, and has a low vapor pressure that
limits its losses to the treated gas. The solvent has a high solubility for carbon dioxide,
hydrogen sulfide, and carbonyl sulfide. It also has appreciable selectivity for hydrogen
sulfide over carbon dioxide. The process can be configured in various ways, depending on
the requirements for the level of hydrogen sulfide/carbon dioxide selectivity, the depth of
sulfur removal, the need for bulk carbon dioxide removal, and whether the gas needs to
be dehydrated (Fig. 2.8). The gas stream from the low-pressure flash is combined with the
acid gas from the regenerator. This combined gas stream is then sent to a sulfur recovery
unit. However, the hydrogen sulfide content could be too low for use in a conventional
Claus plant.
Acid gas
Clean gas
Feed gas
Absorber
Regenerator
FIGURE 2.8 The Selexol process.
The Rectisol process is the most widely used physical solvent gas-treating process in
the world. The process uses chilled methanol (methyl alcohol, CH OH) at a temperature of
3
about −40 to −80°F. The selectivity (by methanol) for hydrogen sulfide over carbon dioxide
at these temperatures is about 6:1, a little lower than that of the Selexol process at its usual
operating temperature. However, the solubility of hydrogen sulfide and carbonyl sulfide
in methanol, at typical process operating temperatures, are higher than in Selexol and allow
for very deep sulfur removal. The high selectivity for hydrogen sulfide over carbon dioxide,
combined with the ability to remove carbonyl sulfide, is the primary advantage of the process.