Page 54 - Synthetic Fuels Handbook
P. 54
42 CHAPTER TWO
50
0
C1 C2 C3 C4 C5 C6 C7 C8
–50
FP , °C –100
–150
–200
–250
Carbon number
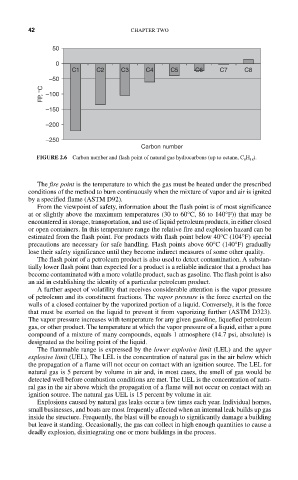
FIGURE 2.6 Carbon number and flash point of natural gas hydrocarbons (up to octane, C 8 H 18 ).
The fire point is the temperature to which the gas must be heated under the prescribed
conditions of the method to burn continuously when the mixture of vapor and air is ignited
by a specified flame (ASTM D92).
From the viewpoint of safety, information about the flash point is of most significance
at or slightly above the maximum temperatures (30 to 60°C, 86 to 140°F)) that may be
encountered in storage, transportation, and use of liquid petroleum products, in either closed
or open containers. In this temperature range the relative fire and explosion hazard can be
estimated from the flash point. For products with flash point below 40°C (104°F) special
precautions are necessary for safe handling. Flash points above 60°C (140°F) gradually
lose their safety significance until they become indirect measures of some other quality.
The flash point of a petroleum product is also used to detect contamination. A substan-
tially lower flash point than expected for a product is a reliable indicator that a product has
become contaminated with a more volatile product, such as gasoline. The flash point is also
an aid in establishing the identity of a particular petroleum product.
A further aspect of volatility that receives considerable attention is the vapor pressure
of petroleum and its constituent fractions. The vapor pressure is the force exerted on the
walls of a closed container by the vaporized portion of a liquid. Conversely, it is the force
that must be exerted on the liquid to prevent it from vaporizing further (ASTM D323).
The vapor pressure increases with temperature for any given gasoline, liquefied petroleum
gas, or other product. The temperature at which the vapor pressure of a liquid, either a pure
compound of a mixture of many compounds, equals 1 atmosphere (14.7 psi, absolute) is
designated as the boiling point of the liquid.
The flammable range is expressed by the lower explosive limit (LEL) and the upper
explosive limit (UEL). The LEL is the concentration of natural gas in the air below which
the propagation of a flame will not occur on contact with an ignition source. The LEL for
natural gas is 5 percent by volume in air and, in most cases, the smell of gas would be
detected well before combustion conditions are met. The UEL is the concentration of natu-
ral gas in the air above which the propagation of a flame will not occur on contact with an
ignition source. The natural gas UEL is 15 percent by volume in air.
Explosions caused by natural gas leaks occur a few times each year. Individual homes,
small businesses, and boats are most frequently affected when an internal leak builds up gas
inside the structure. Frequently, the blast will be enough to significantly damage a building
but leave it standing. Occasionally, the gas can collect in high enough quantities to cause a
deadly explosion, disintegrating one or more buildings in the process.