Page 22 - Thermodynamics of Biochemical Reactions
P. 22
1.6 pKs of Weak Acids 15
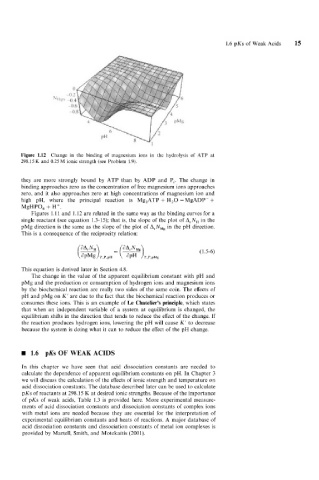
Figure 1.12 Change in the binding of magnesium ions in the hydrolysis of ATP at
298.1 5 K and 0.25 M ionic strength (see Problem 1.9).
they are more strongly bound by ATP than by ADP and Pi. The change in
binding approaches zero as the concentration of free magnesium ions approaches
zero, and it also approaches zero at high concentrations of magnesium ion and
high pH, where the principal reaction is Mg,ATP + H,O = MgADP- +
MgHPO, + H’.
Figures 1.11 and 1.12 are related in the same way as the binding curves for a
single reactant (see equation 1.3-15); that is, the slope of the plot of ArNH in the
pMg direction is the same as the slope of the plot of ArNMg in the pH direction.
This is a consequence of the reciprocity relation:
(1.5-6)
This equation is derived later in Section 4.8.
The change in the value of the apparent equilibrium constant with pH and
pMg and the production or consumption of hydrogen ions and magnesium ions
by the biochemical reaction are really two sides of the same coin. The effects of
pH and pMg on K’ are due to the fact that the biochemical reaction produces or
consumes these ions. This is an example of Le Chatelier’s principle, which states
that when an independent variable of a system at equilibrium is changed, the
equilibrium shifts in the direction that tends to reduce the effect of the change. If
the reaction produces hydrogen ions, lowering the pH will cause K‘ to decrease
because the system is doing what it can to reduce the effect of the pH change.
H 1.6 pKs OF WEAK ACIDS
In this chapter we have seen that acid dissociation constants are needed to
calculate the dependence of apparent equilibrium constants on pH. In Chapter 3
we will discuss the calculation of the effects of ionic strength and temperature on
acid dissociation constants. The database described later can be used to calculate
pKs of reactants at 298.15 K at desired ionic strengths. Because of the importance
of pKs of weak acids, Table 1.3 is provided here. More experimental measure-
ments of acid dissociation constants and dissociation constants of complex ions
with metal ions are needed because they are essential for the interpretation of
experimental equilibrium constants and heats of reactions. A major database of
acid dissociation constants and dissociation constants of metal ion complexes is
provided by Martell, Smith, and Motekaitis (2001).