Page 18 - Thermodynamics of Biochemical Reactions
P. 18
1.4 Apparent Equilibrium Constants of Biochemical Reactions 11
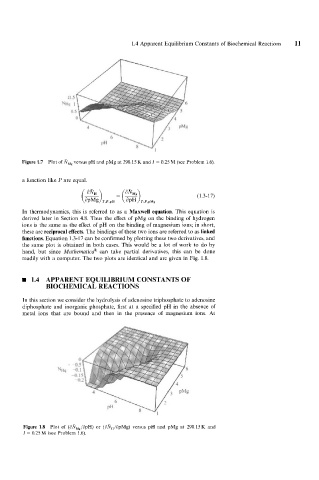
Figure 1.7 Plot of NMg versus pH and pMg at 298.15 K and I = 0.25 M (see Problem 1.6).
a function like P are equal.
( 1.3- 17)
In thermodynamics, this is referred to as a Maxwell equation. This equation is
derived later in Section 4.8. Thus the effect of pMg on the binding of hydrogen
ions is the same as the effect of pH on the binding of magnesium ions; in short,
these are reciprocal effects. The bindings of these two ions are referred to as linked
functions. Equation 1.3-17 can be confirmed by plotting these two derivatives, and
the same plot is obtained in both cases. This would be a lot of work to do by
hand, but since Mathematica' can take partial derivatives, this can be done
readily with a computer. The two plots are identical and are given in Fig. 1.8.
1.4 APPARENT EQUILIBRIUM CONSTANTS OF
BIOCHEMICAL REACTIONS
In this section we consider the hydrolysis of adenosine triphosphate to adenosine
diphosphate and inorganic phosphate, first at a specified pH in the absence of
metal ions that are bound and then in the presence of magnesium ions. At
Figure 1.8 Plot of (aN,,/apH) or (am,/apMg) versus pH and pMg at 298.15K and
I = 0.25 M (see Problem 1.6).