Page 232 - Thermodynamics of Biochemical Reactions
P. 232
232 Mathernatica Solutions to Problems
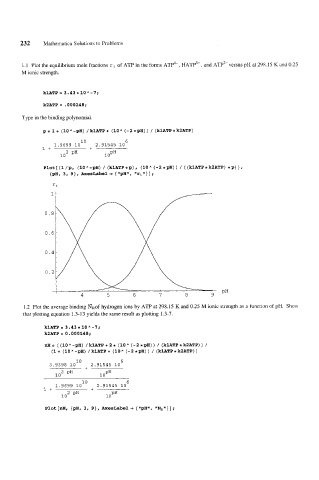
1.1 Plot the equilibrium mole fractions r, of ATP in the forms ATP4-, HATP3-, and ATP2-versus pH at 298.15 K and 0.25
M ionic strength.
klATP = 3 -43 * 10 " -7;
k2ATP = .000148;
Type in the binding polynomial.
B= 1+ (10"-gH) /klATP+ (10" (-2*gH)) / (klATP*k2ATP)
10 6
1.9699 10 2.91545 10
1+ +
lo2 pH 1 OPH
PlOt[{l/g, (lOA-gH) / (klATP*p), (10" (-2*gH)) / ((klATP*k2ATP) *p)},
{pH, 3, 9}, AxesLabel + {"pH", "ri"}] ;
r-
4 5 6 7 8
1.2 Plot the average binding NHof hydrogen ions by ATP at 298.15 K and 0.25 M ionic strength as a function of pH. Show
that plotting equation 1.3-13 yields the same result as plotting 1.3-7.
klATP = 3.43 * 10 " -7;
k2ATP=0.000148;
nH= ((lOA-gH) /klATP+2* (10A(-2*gH)) / (klATP*k2ATP)) /
(1+ (10"-pH) /klATP+ (10" (-2*gH)) / (klATP*kSATP))
6
3.9398 10 lo 2.91545 10
lo2 pH 1 OPH
10 6
1.9699 10 2.91545 10
1r A
I ,
2 PH
10 1 OPH
Plot [nH, {gH, 3, 93, AxesLabel + { ampH", llNHtn} ] ;