Page 234 - Thermodynamics of Biochemical Reactions
P. 234
234 Mathematica Solutions to Problems
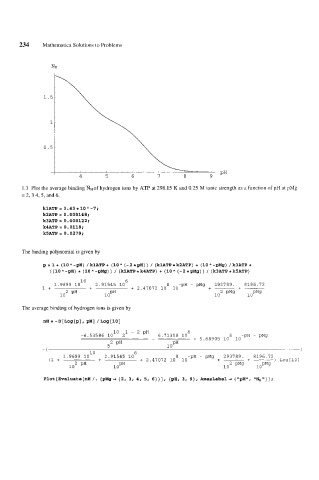
1.3 Plot the average binding WHof hydrogen ions by ATP at 298.15 K and 0.25 M ionic strength as a function of pH at pMg
= 2, 3 4, 5, and 6.
klATP = 3.43 * 10 " -7;
k2ATP= 0.000140;
k3ATP=0.000122;
k4ATP=0.0110;
k5ATP=O.O279;
The binding polynomial is given by
g= 1+ (10A-gH) /klATP+ (10" (-2*pH)) / (klATP*k2ATP) + (10"-gMg) /k3ATP+
((IOA-gH) * (lO"-pMg)) / (klATP*k4ATP) + (10" (-2*PMg)) / (k3ATP*kSATP)
10 6
1.9699 10 2.91545 10 8 -pH - pMg 293789. 8196.72
1+ + + 2.47072 10 10 +-+-
lo2 pH loPH 102 PMg 1 OPMg
The average binding of hydrogen ions is given by
nH = -D[Log[g], pH] /Log[lO]
10 1 - 2 pH 6
-4.53586 10 2 6.71308 10 8 -pH - pMg
- - 5.68905 10 10
52 PH
1 OPH
1u b
1.9699 10 + 2.91545 10 8 -pH - pMg 293789. 8196.72
(1 + + 2.47072 10 10 +-+- ) Log[lOl
102 PMg
lo2 pH 1 OPH 1 OPMg
Plot[Evaluate[nH/. [pMg+ [2, 3, 4, 5, 6}}], [pH, 3, 9}, AxesLabel+ ["pH", llNH"}];